CRISPR-Cas9 represents one of the most impactful discoveries in the field of genetic engineering and medicine of recent times. Its discovery catalyzed a paradigm shift in the treatment approach to many genetic diseases, paving the way to simple and effective gene therapy for a large class of diseases. In fact, the FDA recently approved the first CRISPR-Cas9 therapy on December 8, 2023.
Despite its popularity and widespread use in various research areas, many scientific reports have highlighted potential risks and safety concerns due to high frequency off-target editing in human cells. Those reports sparked an intense research effort towards developing novel strategies to enhance the fidelity of the CRISPR-Cas9 gene editing system.
Despite the progressive advancements in CRISPR-Cas9 technology, crucial questions about the molecular mechanisms driving off-target gene editing have remained elusive. The complexity of dynamic molecular interactions, particularly under varied cellular conditions, have prevented scientists from a full understanding of mechanism of off-target events.
A recent groundbreaking collaborative study from Imperial College London, AstraZeneca, Francis Crick Institute, University College London, MRC-LMS, and Cardiff University (Newton MD et al. Molecular Cell, 2023) utilizing LUMICKS C-Trap technology, has unraveled a new crucial insight, shining new light into the molecular mechanism of off-target gene editing.
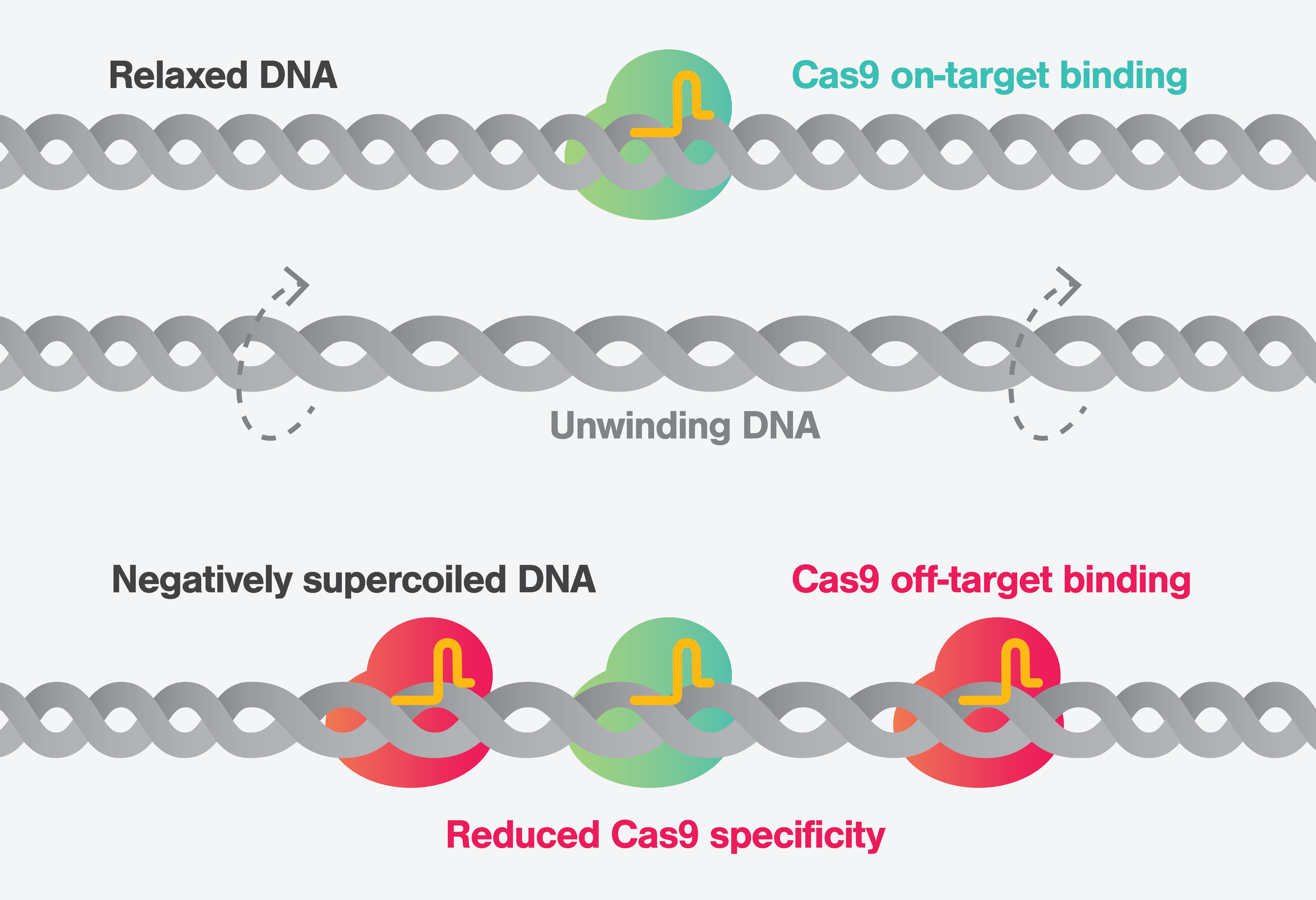
The work demonstrates a previously unappreciated role of DNA supercoiling in influencing dramatically CRISPR-Cas9 specificity and its off-target activity. This approach provided a more in-depth understanding of how DNA’s structural form and alterations impacts CRISPR-Cas9’s targeting accuracy and drive off-target mutagenesis.
Interesting fact:
Negative supercoiling induces Cas9 off-target binding and cleavage, reducing specificity and resulting in the potential for cleavage at over 10,000 sites across the human genome.
In the experiment, researchers successfully mimicked the natural conditions of DNA under cellular stress, revealing that negative supercoiling significantly increases CRISPR-Cas9’s off-target activity. This finding is a significant change from what was previously known about the CRISPR-Cas9 and opens new strategies for research in gene editing and protein engineering aimed at developing novel protein variants with improved performances and fidelity against DNA structural alterations.
Understanding critical insights of CRISPR-Cas9 interaction with DNA under various conditions is crucial for the advancement of safe and effective gene therapies. Additionally, the research underscores the potential for more precise applications of CRISPR-Cas9 in treating genetic disorders. By acknowledging the influence of DNA supercoiling, scientists can develop more targeted strategies to mitigate off-target effects, enhancing the safety and efficacy of gene therapies.
This study specifically highlights the need for more refined approaches in gene editing, especially considering the complexities of the human genome and the continuous structural changes which is subject to due to the various cellular processes such as DNA transcription that subjects the DNA to a considerable level of structural stress, impacting the ability of gene editing tools to be effective, precise and safe.
This study also serves as an example of the power of collaborative research and the use of cutting-edge technology like the LUMICKS C-Trap in advancing the scientific understanding of complex biological systems such as CRISPR-Cas9.
Discover how dynamic single-molecule methods reveal crucial insights into molecular processes here
Newton et al., 2023, Molecular Cell 83, 3533–3545 October 5, 2023
Negative DNA supercoiling induces genome-wide Cas9 off-target activity.