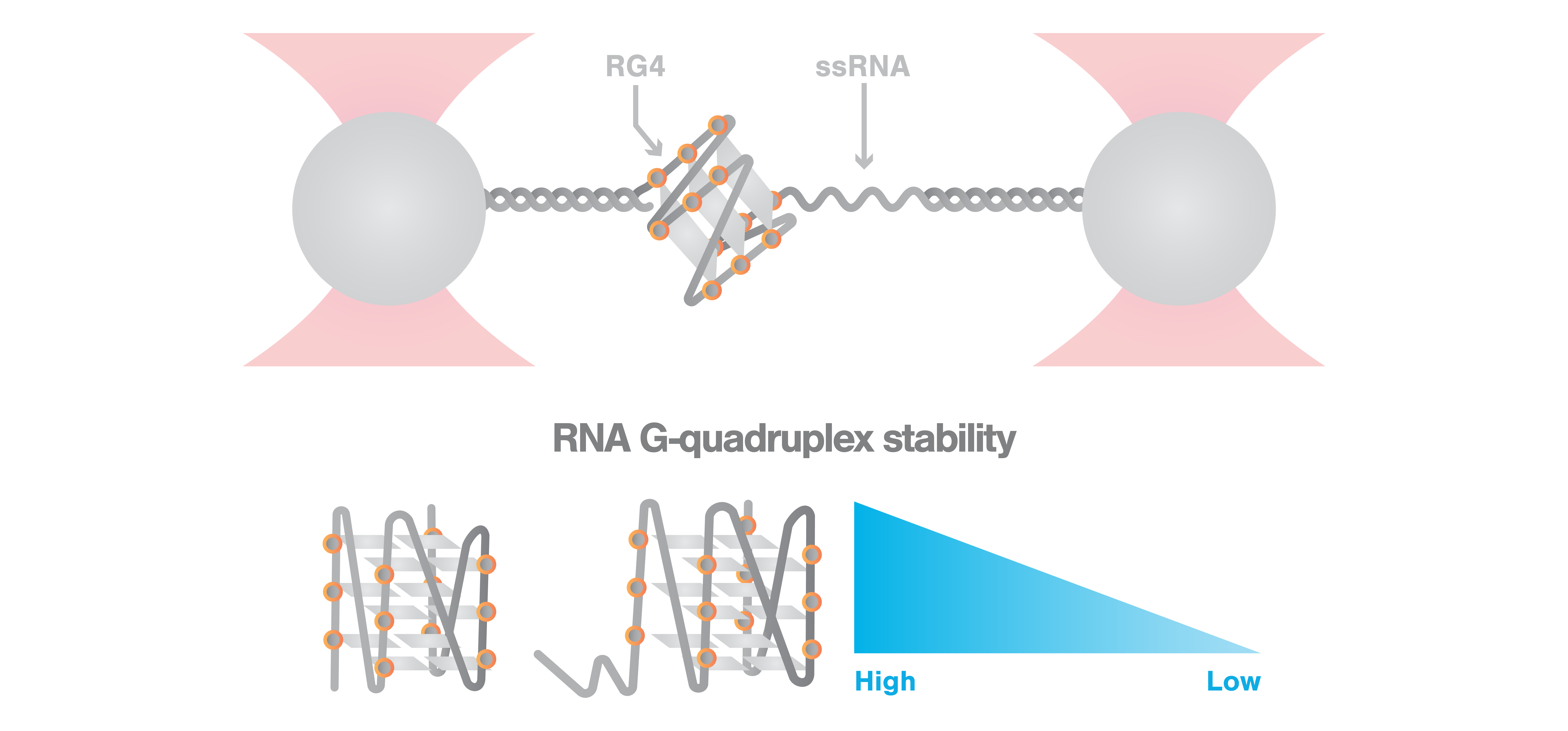
A recent article published in the Journal of Physical Chemistry Letters provides insight into the stability and folding dynamics of RNA G-quadruplexes (RG4). Using the C-Trap® optical tweezers, Ye et al. investigated the influence of a proximal single-stranded (ss) RNA sequence and found that its existence significantly impairs RG4 stability. Other methods including NMR, single-molecule FRET, and a cleavage assay contributed to the data showing that a minimum of six ssRNA nucleotides is required for this destabilizing effect, leading to at least three potential intermediate RG4 conformers.
Intramolecular RG4 motifs play important roles in cellular functions like gene expression and telomere homeostasis. Contradictory findings on whether these structures occur in a folded or an unfolded state in vivo leave open questions that could however be explained by the existence of structural polymorphism. Thus, understanding the stability and dynamics of RG4 is highly important.
In this study, Ye et al. studied a RG4-forming sequence that is found at the end of human telomerase RNA, in the presence and absence of a proximal ssRNA. To test the mechanical stability of both structures, the scientists employed C-Trap optical tweezers and attached the constructs via dsDNA tethers to the optically trapped beads, allowing manipulation of the complex and applied tension The high control of the instrument allowed a step-wise extension of the structures with a precise speed of 10 nm/s. This resulted in unfolding of the RG4 motifs with rupture forces of ∼ 24.4 pN for RG4 with ssRNA and of ∼ 32.4 pN for RG4 without ssRNA, showing that the proximal ssRNA weakens RG4 mechanical stability.
These observations led the authors to hypothesize that the less stable structure would allow easier dismantling by motor proteins achieving higher accessibility of G-rich sequence. Employing an endonuclease cleavage assay, Ye et al. confirmed their hypothesis.
This study highlights the value of combining different experimental methods with dynamic single-molecule experiments. The findings from the single-molecule assays using the C-Trap offer direct proof that the stability of RG4 complexes is lowered in the presence of ssRNA protrusions. The presented data also provides insights into the dynamics of RG4 and its conformational transitions, which are crucial for the regulatory function of RNA molecules in vivo.
Read the full article titled “Proximal Single-Stranded RNA Destabilizes Human Telomerase RNA G-Quadruplex and Induces Its Distinct Conformers” in the Journal of Physical Chemical Letters.