A recent publication by Kucera et al. in Nature Communications combined TIRF assays and Optical Tweezers measurements to investigate myosin-independent actin contraction that is relevant during cytokinesis and cell division. The authors found a new mechanism showing how anillin, a non-motor actin crosslinking and scaffolding protein, substitutes for the activity of molecular motors to generate contractile forces in actin networks. Specifically, anillin diffuses in one direction over actin maximizing the overlap between bundled actin filaments while generating contractile forces of tens of pico-Newtons.
The process of cell division of most eukaryotic cells is driven by the constriction of the cytokinetic contractile ring which consists of actin filament bundles overlapping in mixed directions. Earlier hypotheses suggested that myosin motors are responsible for the contraction of the ring. However, due to the disordered nature of the actin filament orientation, myosin could only affect the local sliding of the actin, leading to the requirement of another mechanism to coordinate the full ring contraction. Additionally, myosin-independent phases of the contraction as well as organisms that lack myosin II further suggest the involvement of other factors.
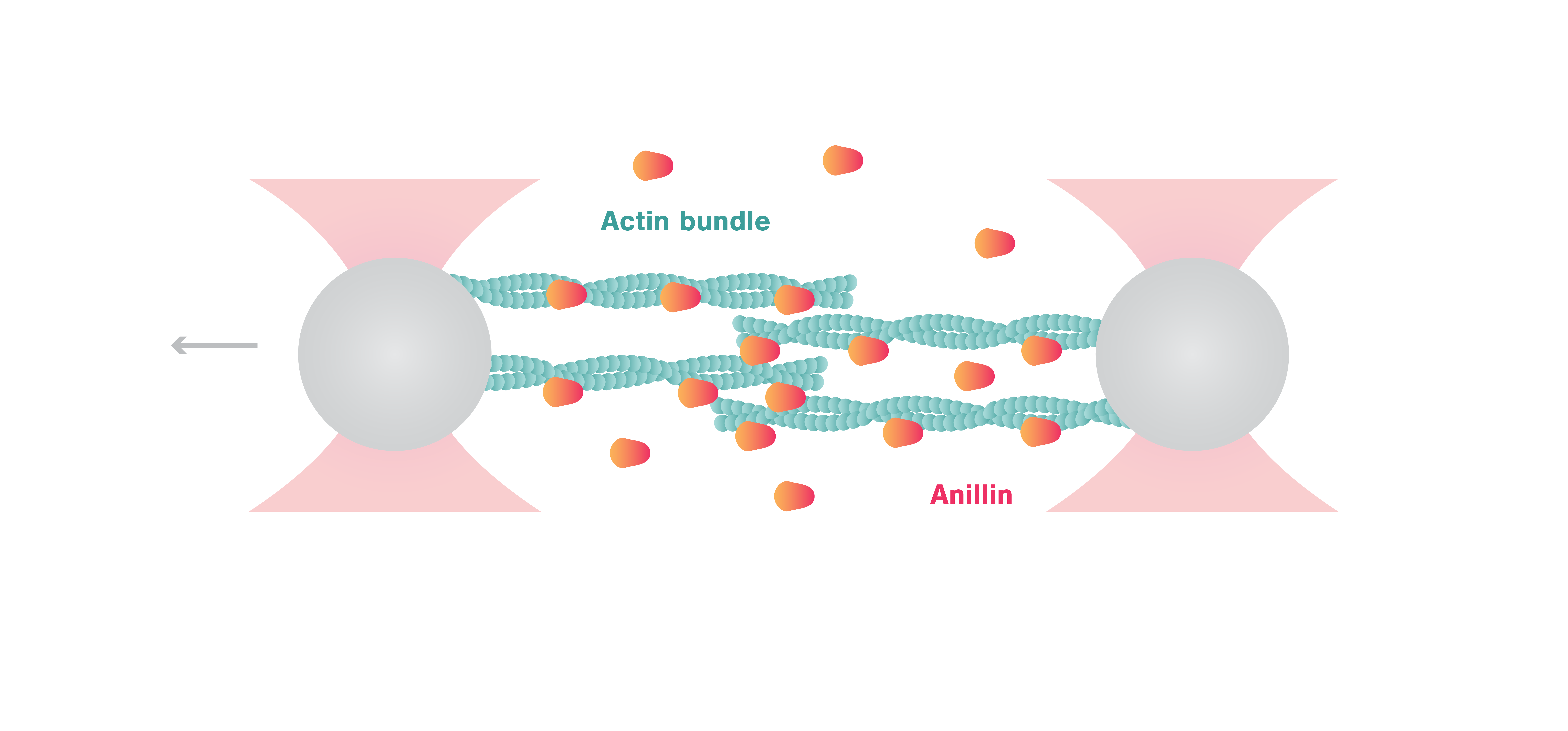
In this article, Kucera et al. provide direct experimental evidence for the autonomous contraction of actin by anillin, without the presence of myosin. Sliding assays observed by TIRF showed the unidirectional diffusion of anillin along actin leading to contraction of bundled actin filaments. Using the C-Trap® Optical Tweezers, the authors connected these observations to the mechanical function of anillin.
By attaching actin filaments to two optically-trapped beads, the scientists created crosslinked actin bundles consisting of ~ 5 filaments between the beads. The C-Trap enabled a step-wise movement of the beads resulting in controlled extension of the anillin-actin complex. Using this assay, Kucera et al. showed that anillin generates forces (10 pN at 25 nM anillin concentration) that are comparable to myosins and are thus relevant for the ring contraction process. Interestingly, further experiments determined that anillin-generated forces were inversely proportional to the length of the overlap of actin bundles and proportional to the density of anillin retained in the overlap.
This study presents new insights into the mechanism of cytokinesis, showing anillin crosslinker as an autonomous factor achieving actin contractility without the necessity of myosin motors. Combining TIRF assays and C-Trap analysis provides direct proof of anillin force generation thus drawing a more detailed picture of the ring contraction mechanism and highlighting the value that optical tweezer experiments bring to answering biological research questions.
To see all results, read the full article titled “Anillin propels myosin-independent constriction of actin rings” published in Nature Communications.