A recent work published in eLife by Francisco de Asis Balaguer et al. shed light on the interaction of ParB with DNA, the consequent DNA condensation, and the role of cytidine triphosphate (CTP) in this process – at a single-molecule level. Using the C-Trap® the researchers investigated the role of CTP binding in the critical interaction between centromere-like parS DNA sequences and the ParB protein.
ParB is a DNA-binding protein and a member of the ParABS complex, which is in charge of separating sister chromosomes during cell replication in bacteria. It has been established by previous studies that ParB could specifically bind to a centromere-like palindromic sequence named parS. After ParB binding to parS DNA sequence, DNA-bound ParBs self-associate, bind to distal DNA segments, and create a network leading to the formation of DNA bridges and consequent DNA condensation. Prior studies have found that CTP is important for ParB spreading on DNA, but the role of CTP-dependent spreading of ParB in DNA condensation was not clear.
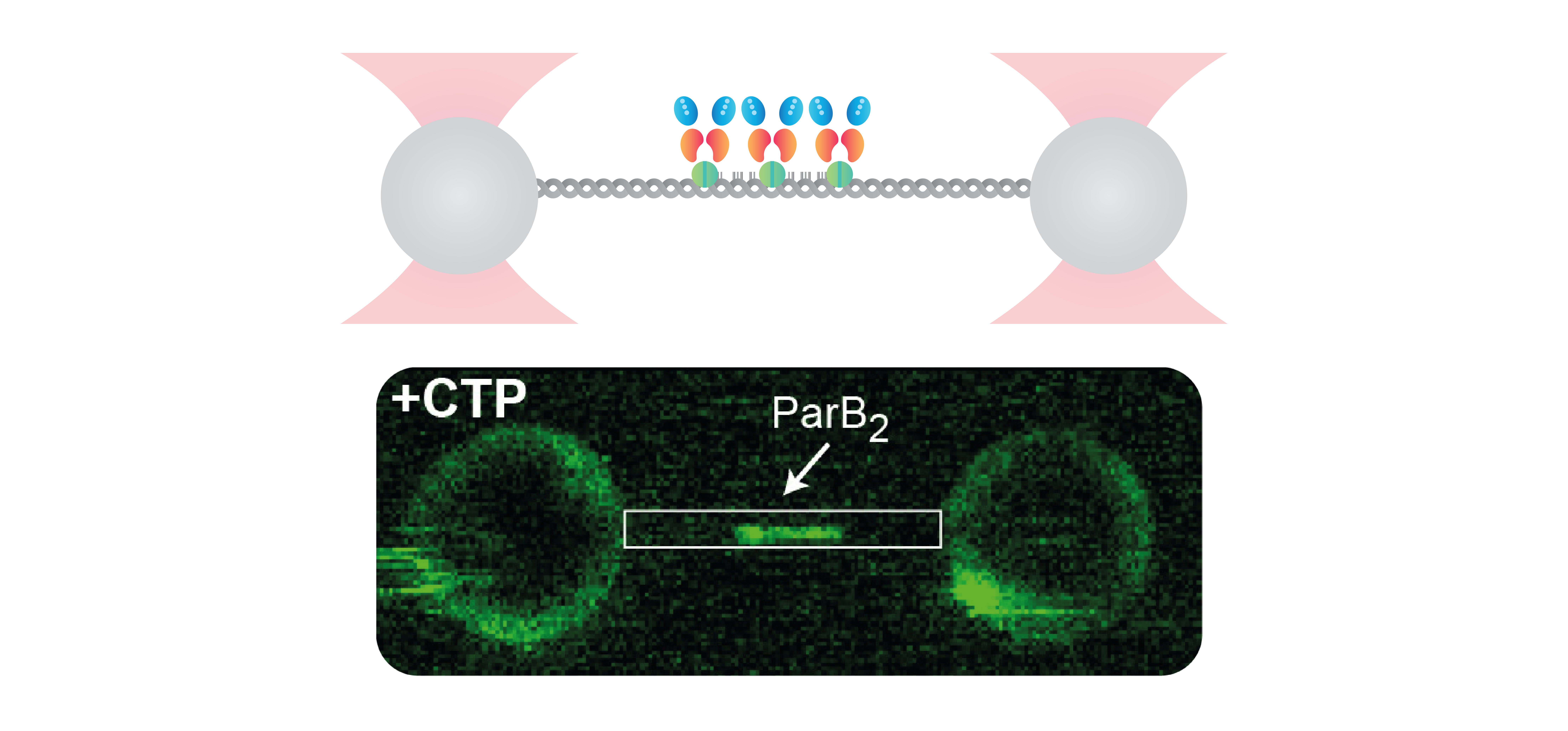
To gain insight into this process, Francisco de Asis Balaguer and co-workers visualized individual DNA molecules containing several parS sequences while incubating it with fluorescently labeled ParB – in the presence and absence of CTP. Their results showed that ParB can bind to the parS sequence in the absence of CTP, but that CTP presence is necessary to induce a conformational change in ParB and allow it to spread outside of parS and into non-specific DNA (nsDNA) regions. Importantly, ParB proteins appear at a much lower density at nsDNA and were only detected on DNA filaments containing parS sequences – suggesting that ParB requires loading via parS and from there slides into non-specific regions. By placing DNA-binding proteins as “roadblocks” that presented an obstacle to ParB movement, the researchers could confirm that ParB, in presence of CTP-Mg2+, spreads along the DNA from parS sites in a one-dimensional manner, inducing DNA condensation. This assay was achieved by a sequential assembly of the individual components that was possible utilizing the incorporated microfluidics system of the C-Trap.
Only by employing C-Trap optical tweezers – which enables holding the DNA molecule at a high force and thus preventing ParB-induced DNA condensation – the researchers were able, for the very first time, to directly observe the diffusion of ParB over kilo-base pair distances.
“Here, we used a combination of optical tweezers and confocal microscopy that allowed us not only to observe more precisely the direct binding of ParB to single DNA molecules but also facilitated the quantification of fluorescence intensity around parS sites and distal non-specific sites. “ – the authors write in their article.
This work provides direct evidence of ParB dynamics on DNA and proposes a new working model: ParB-CTP-Mg2+ first loads to parS sites and then diffuses to non-parS regions. There it self-associates and leads to the condensation of kilobase-pair long DNA molecules.
For further details, read the full article published on eLife and titled: CTP promotes efficient ParB-dependent DNA condensation by facilitating one-dimensional diffusion from parS.