What is STED nanoscopy?
Stimulated emission depletion (STED) is a fluorescence microscopy technique that facilitates visualization of sub-cellular organization with unprecedented detail. Stefan Hell, the person who created STED, got the Nobel Prize in Chemistry in 2014 for this invention. STED can achieve super-resolution, bypassing the diffraction limitations of confocal microscopy. It does this by a clever technique that extends upon the basis of confocal microscopy.
Just as in confocal microscopy, fluorophores are excited using a laser beam. With STED, an extra beam with a zero-intensity spot in the middle is centered at the excitation beam to depress the majority of the fluorophores back to the ground state. This is called stimulated emission. Because of the zero-intensity spot, fluorophores in the middle of the excitation beam are not affected by the STED beam. These fluorophores will emit light spontaneously, while the fluorophores affected by the STED beam will emit light stimulation. And here comes the clever trick. Because both emissions possess slightly different wavelengths, they can be separated. Only the spontaneously emitted fluorescence signal is allowed to pass through a filter, while the rest is not.
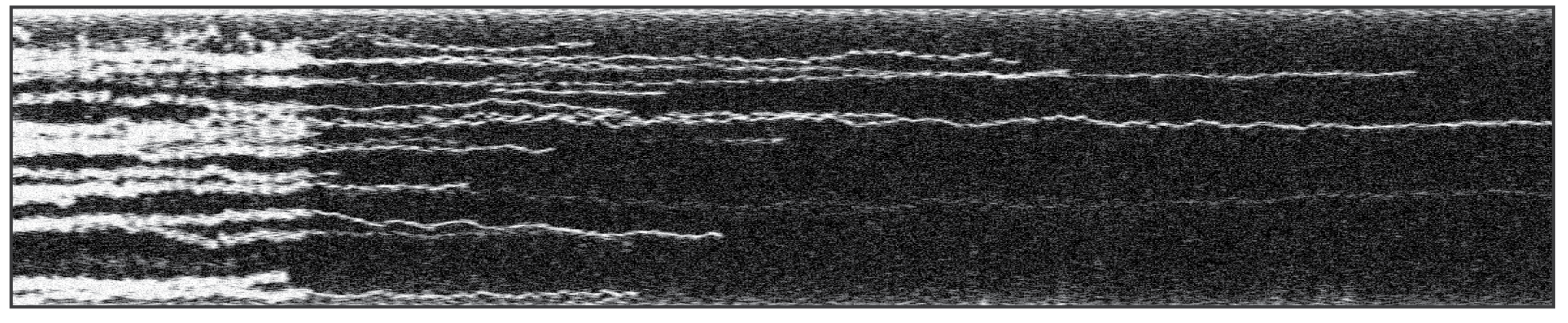
The kymograph above shows the fast dynamics of TFAM proteins on DNA at high protein density. The kymograph was recorded using two methods: confocal (left) and STED (right). The use of STED enabled the tracking of individual protein trajectories, including (un)binding and oligomerization events, which were not always observable with the diffraction-limited resolution of the confocal microscopy.
Explore other imaging techniques
Widefield
Image biomolecular processes with low protein concentration in solution with high acquisition rates.
Confocal Fluorescence
Multicolor confocal, perfect for visualizing biological processes in solution.
TIRF Fluorescence
Suitable for visualization on surfaces as it eliminates background fluorescence outside the focal plane.
IRM (label-free)
IRM allows you to visualize microtubules without the need for fluorescence labeling.
References
1. Hell et al. (1994) Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Optics Letters
2. Heller et al. (2013) STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA. Nature Methods
Our solution
The C-Trap® Optical Tweezers – Fluorescence & Label-free Microscopy is the world’s first instrument that allows simultaneous manipulation and visualization of single-molecule interactions in real time. It combines high resolution optical tweezers, fluorescence and label-free microscopy and an advanced microfluidics system in a truly integrated and correlated solution.
The C-Trap offers you a fast workflow to seamlessly catch and manipulate single molecules. The instrument measures their structural changes or interactions while you visualize them in teal time with high spatial and temporal resolution, ultimately offering you a complete and detailed picture of biomolecular properties and interactions.
Curious to learn more?