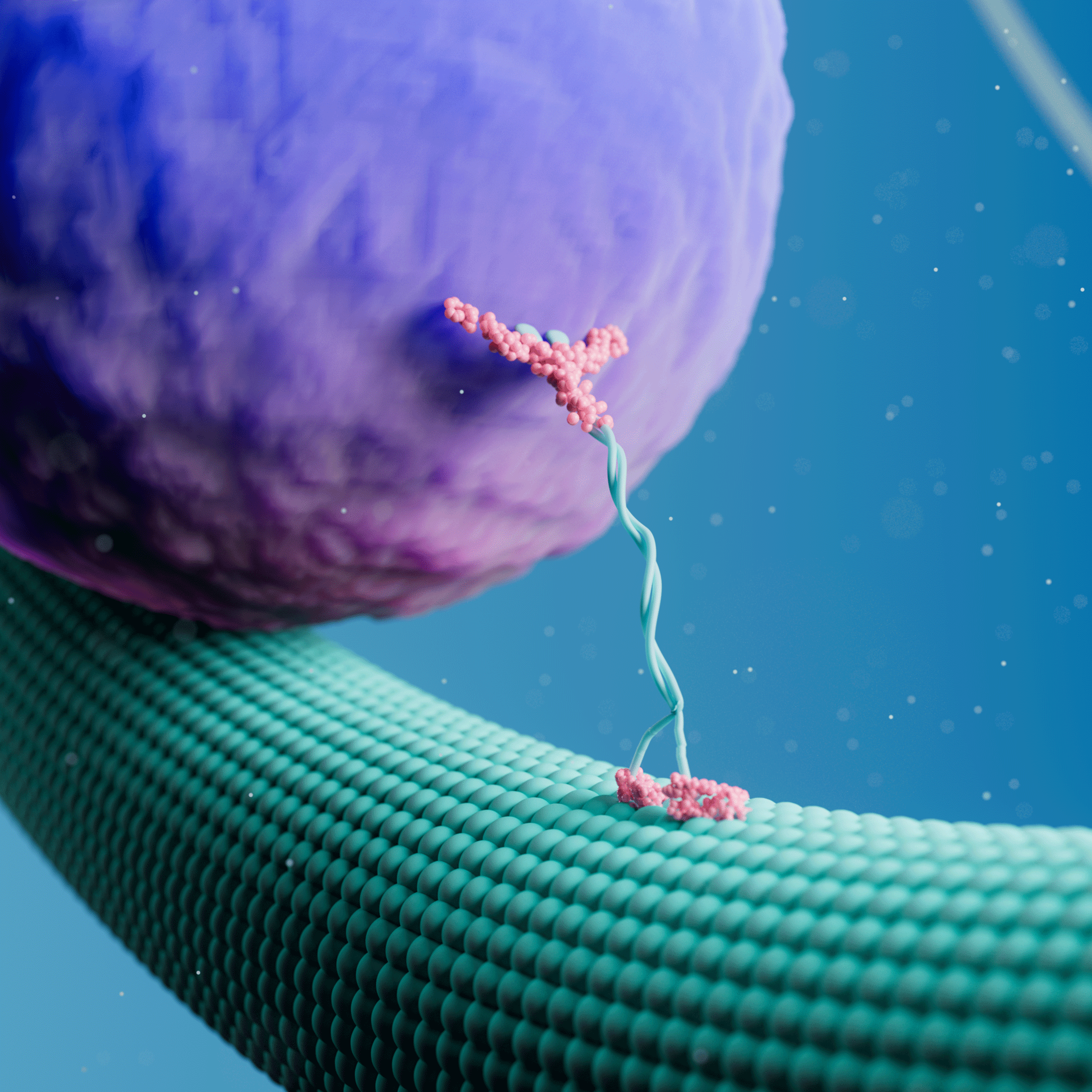
A recent article in Journal of Cell Biology (JCB) describes how researchers at Albert Einstein College of Medicine measured the force-generating properties of KIF1A, a kinesin-3 motor known to transport synaptic vesicle precursors in neurons. Using the C-Trap® optical tweezers and motility assays, the researchers identified the mechanical properties of wild-type and mutant KIF1A and how the motor responds to an external load.
These findings improve our understanding of the mechanical properties of kinesin-3 motors and their competitive relationship with conventional kinesin-1 motors during cargo transport along microtubules. They also highlight how KIF1A-specific mutations associated with neurodegenerative or neurodevelopmental disorders affect the motor’s kinetics.
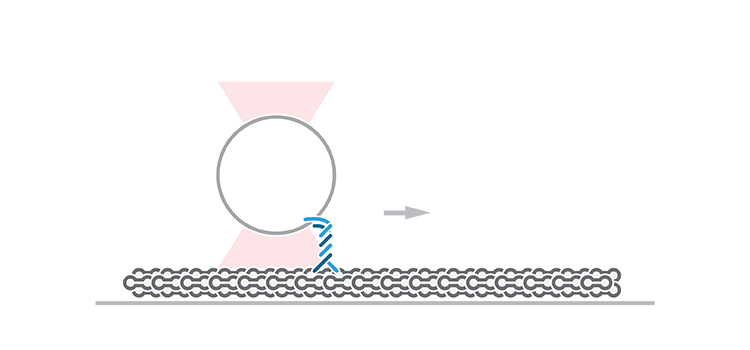
To measure these force-dependent properties of KIF1A, Budaitis et al. tethered a single KIF1A motor to an optically trapped bead and evaluated its response to opposing force loads once attached to a microtubule. The approach revealed how the motor stalls, detaches, and reattaches to a microtubule in response to specific loads. Similar force-generation and mechanical assays have typically been performed on conventional kinesin-1 motors, such as KIF5C, but have never been validated in kinesin-3 motors.
The team found that KIF1A stalled or, more often, detached from single microtubule filaments upon low forces (avg. 2.7 pN). Interestingly, the motor could quickly resume its activity upon immediate reattachment to the microtubule, a property identified to be specifically dependent on the K-loop domain of kinesin-3 motors. Of note, KIF5C detached at higher forces (avg. 4.4 pN) and reattached to a lesser extent than KIF1A.
They next investigated these force-generating properties on mutated KIF1A motors associated with neurodevelopmental and neurodegenerative disorders. Like intact KIF1A motors, the mutant variants also detached from the microtubule before stalling, only to reattach promptly. However, the mutant motors required lower forces to detach from the filament than the intact KIF1A motors. Together with complemental assays, the researchers observed that the mutant variants reduced the motors force generation, processivity, and speed, ultimately impairing cellular cargo transport.
“Our results indicate that these mutations result in reduced speed, processivity, landing rate, and force output of single KIF1A motors and delayed transport driven by teams of mutant motors in an unpolarized cell,” the authors concluded. “It seems likely that, in patients, transport driven by these mutant motors is compromised, given the long distances and spatial constraints that characterize transport in neuronal cells.”
You can read the full article titled “Pathogenic mutations in the kinesin-3 motor KIF1A diminish force generation and movement through allosteric mechanisms” in Journal of Cell biology.