Force-extension, manipulation, and visualization of polymers and protein filaments
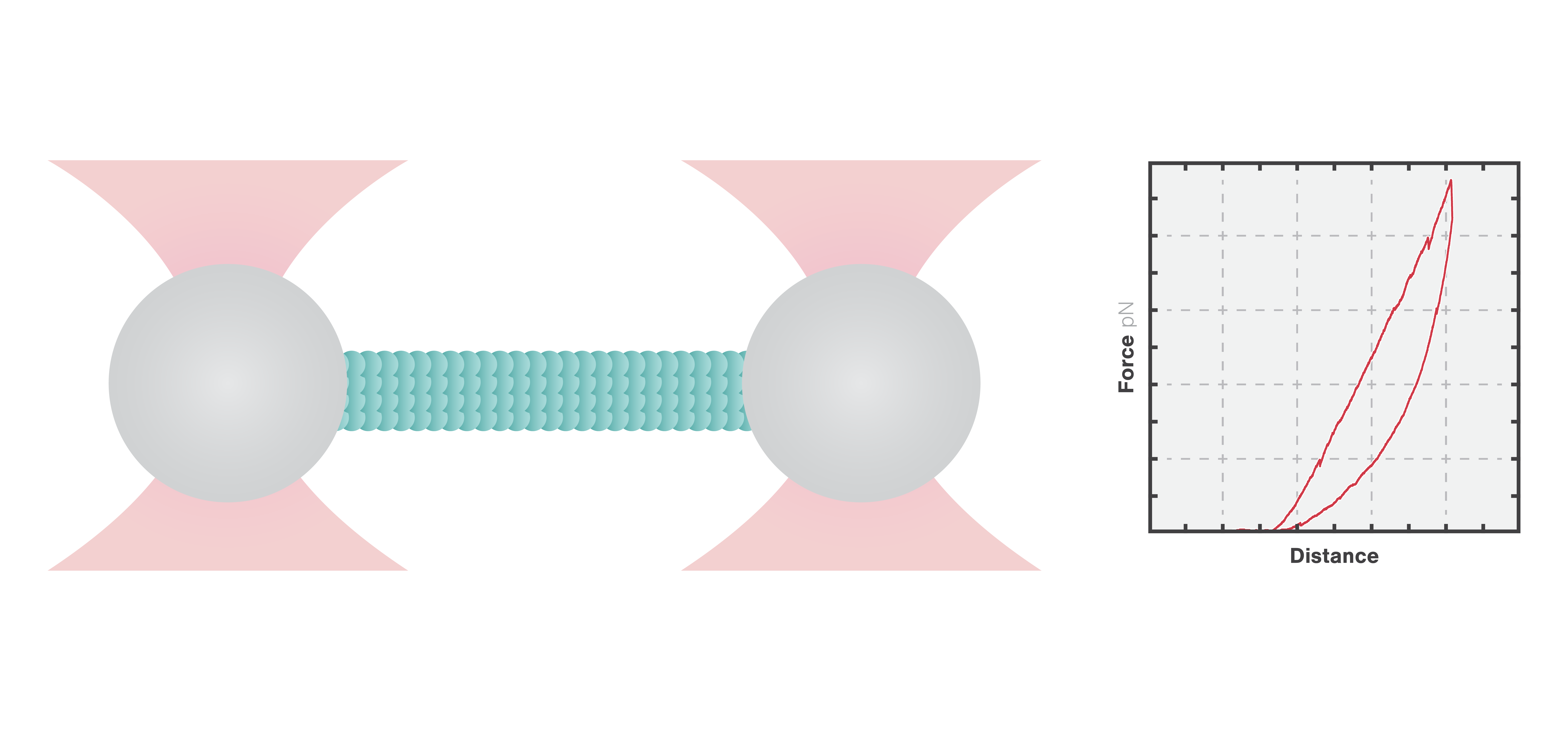
In this experiment, a vimentin filament is tethered between two optically trapped beads. We can obtain the force-distance curve and determine the mechanical properties of the microtubule by simultaneously stretching the filament and measuring the force and the extension.
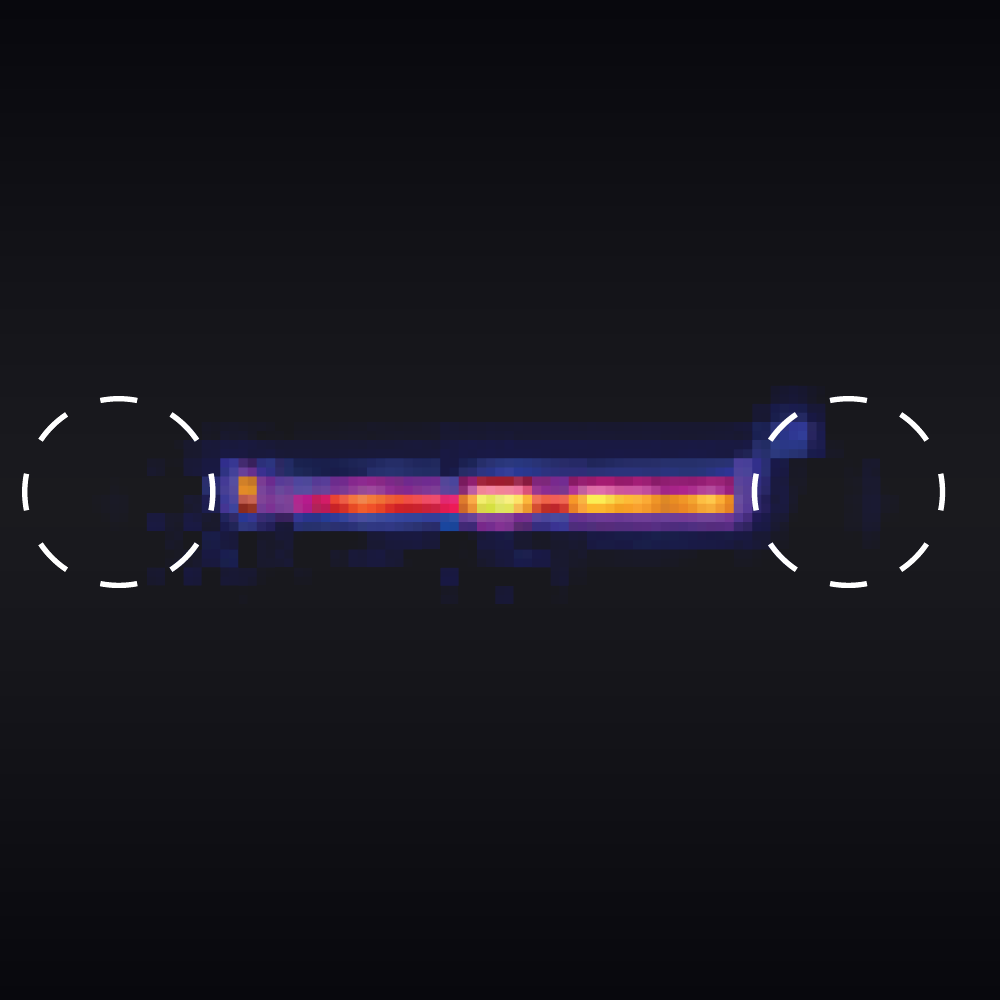
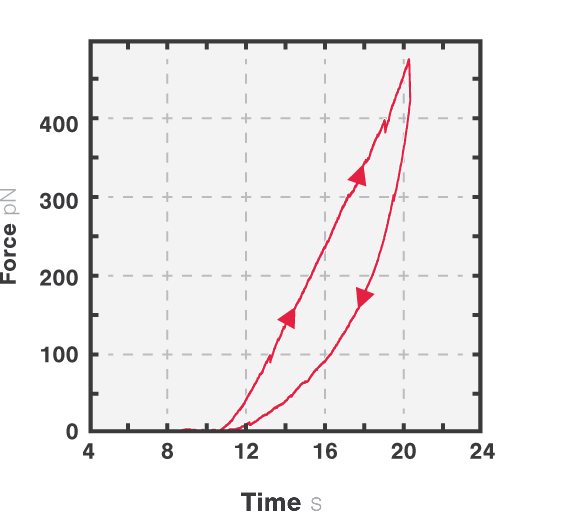
The measured data in Figures 1 and 2 show the extension of a vimentin intermediate filament. The force-distance curve is measured while stretching and relaxing the intermediate filament at a slow speed, allowing for structural equilibrium. The retraction curve shows clear hysteresis due to the remodeling of the vimentin filament under high tension. Simultaneous confocal fluorescence imaging of the vimentin filament is used to resolve this intra-molecular remodeling.
Combining optical tweezers with simultaneous fluorescence measurements allows correlating the mechanical properties of the microtubule with local information.
Label-free observation of microtubules with high sensitivity
In this example, we anchored multiple microtubules to the surface through biotinylated GMPCPP-stabilized seeds. By using Interference Reflection Microscopy (IRM) to image the microtubules in a label-free manner, we observed the microtubules with high sensitivity and contrast (Figure 1).
Sections of the microtubules shown in figure 1 are not rigidly bound to the glass and can freely fluctuate in solution. Distinct patterns composed of alternating light and dark stripes are created by the IRM imaging method. This provides insights into the 3D orientation of the microtubules.
Next, using a C-Trap system equipped with Widefield and IRM we imaged fluorescently-labeled microtubules using both methods (Figure 2).
The new addition of the Surface Assay Toolkit brings fully correlated optical tweezers with IRM and TIRF or Widefield. Couple manipulation and measurements with live observation using different imaging methods and perform truly revolutionary experiments.
Label-free observation of microtubule dynamics
Using optical tweezers correlated with label-free microscopy you can study the dynamics of microtubule filaments to gain insights into the function and regulation of filament assembly. Dynamic microtubule assays can be easily reconstituted in vitro to visualize and measure intricate phenomena like protein binding, microtubule disassembly, severing, and cargo transport.
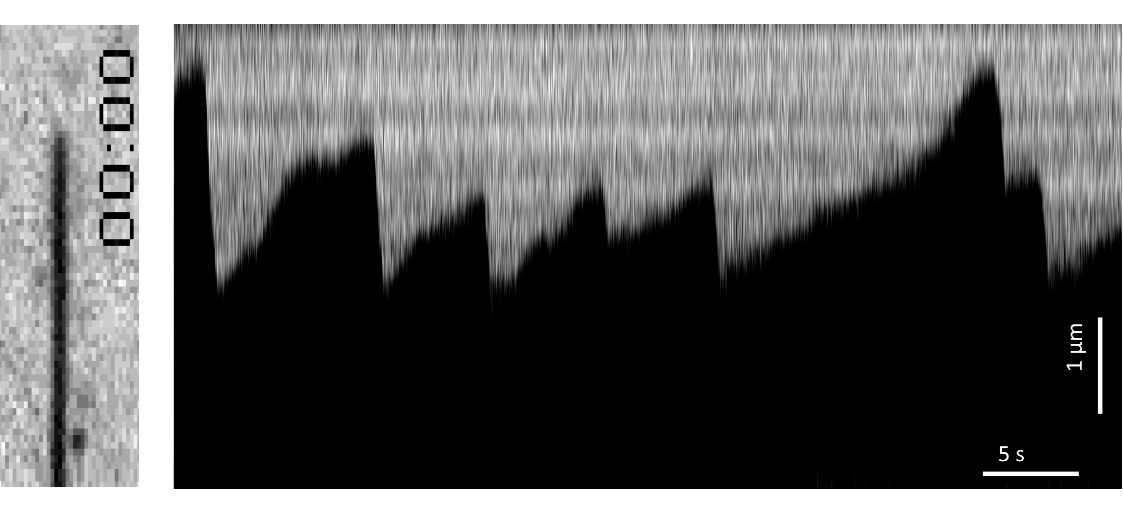
In the same experiment as the one previously described, we anchored multiple microtubules to the surface through biotinylated GMPCPP-stabilized seeds. This time, by using IRM to image the microtubules in a label-free manner, we observed microtubule dynamics over time (Figure 1).
Using this method it becomes possible to measure the force, speed, distance, conversion rate of filament growth and shrinkage, and to detect rare and transient events, which include rescue, catastrophe, and pause.
Manipulation and visualization of microtubules
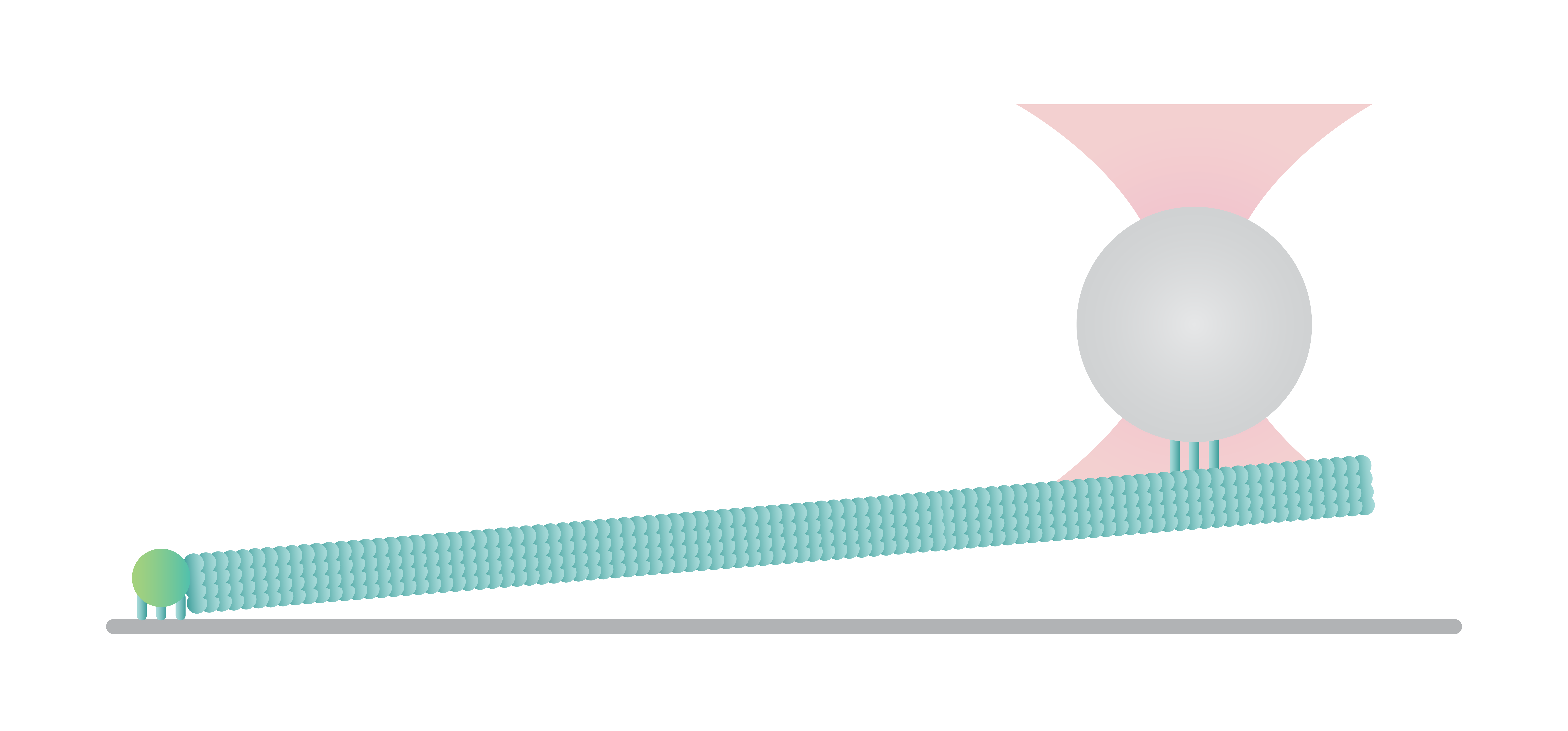
Microtubules can be manipulated to study their mechanical properties, dynamics, and interaction with several microtubule-binding proteins. Here a surface-tethered microtubule is attached to an optically trapped bead for the measurement of growth and shrinkage. In this assay, it is possible to apply force along the direction of the filament and measure precisely force and distance values with sub-pN and sub-nm resolution.
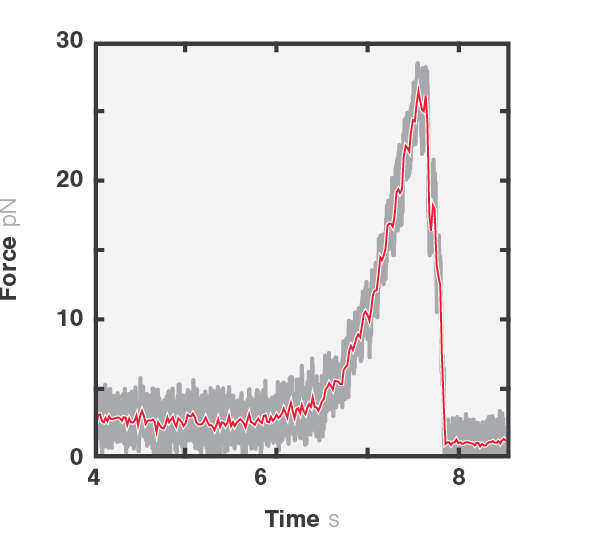
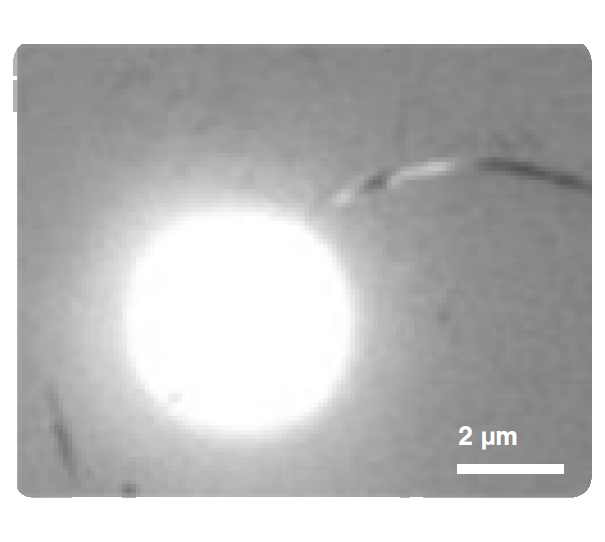
Figure 1 shows the force exerted by a microtubule upon bending (4-6 seconds) and followed by the disruption between the kinesin-coated bead and the filament (6-8 seconds). We can observe the disruption event between the kinesin-coated bead and the filament at a force of ~27 pN. Similar assays can be used to investigate the function and mechanism of various microtubule-associated proteins (MAPs), such as microtubule plus-end-tracking proteins (+TIPs) or (fluorescence) kinetochore particles.
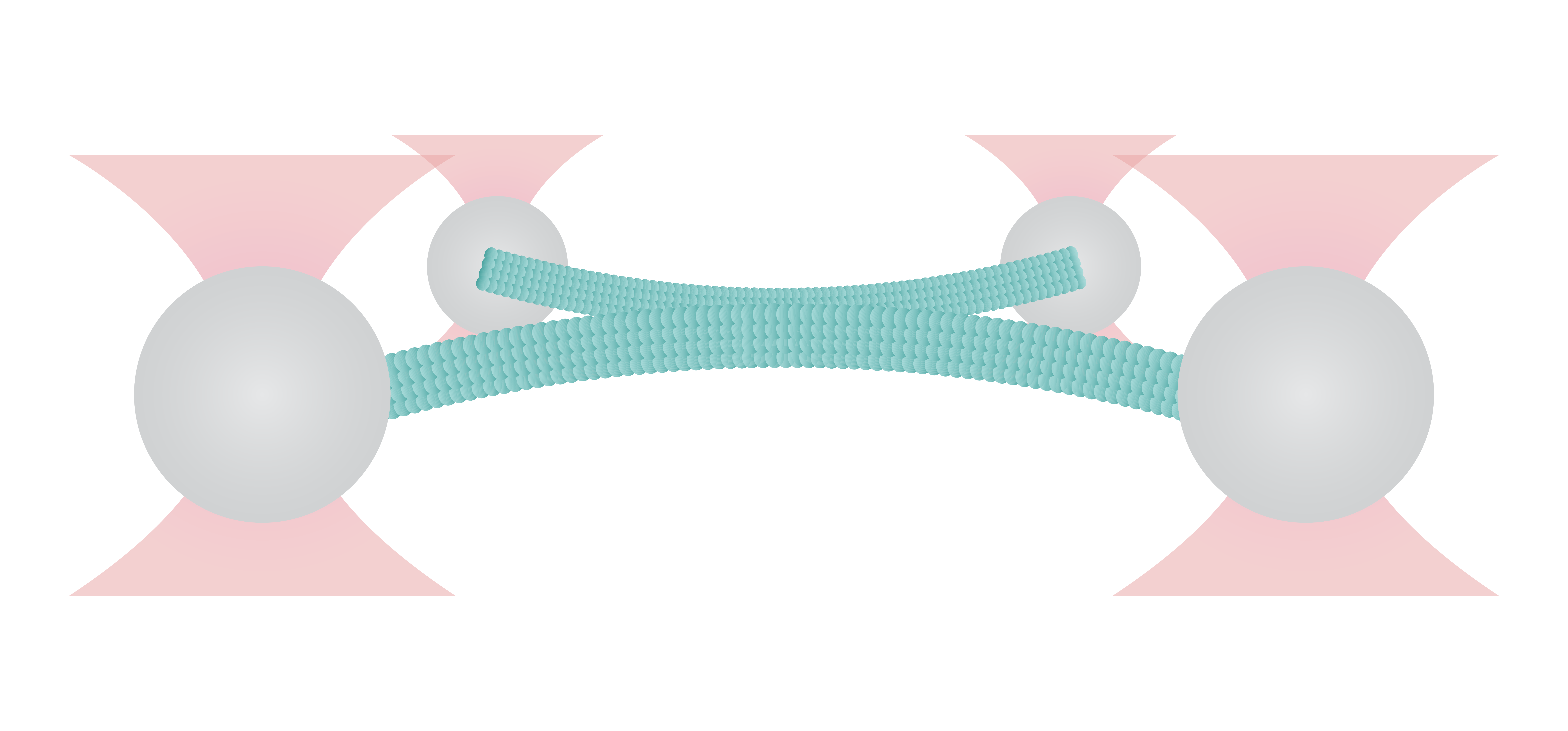
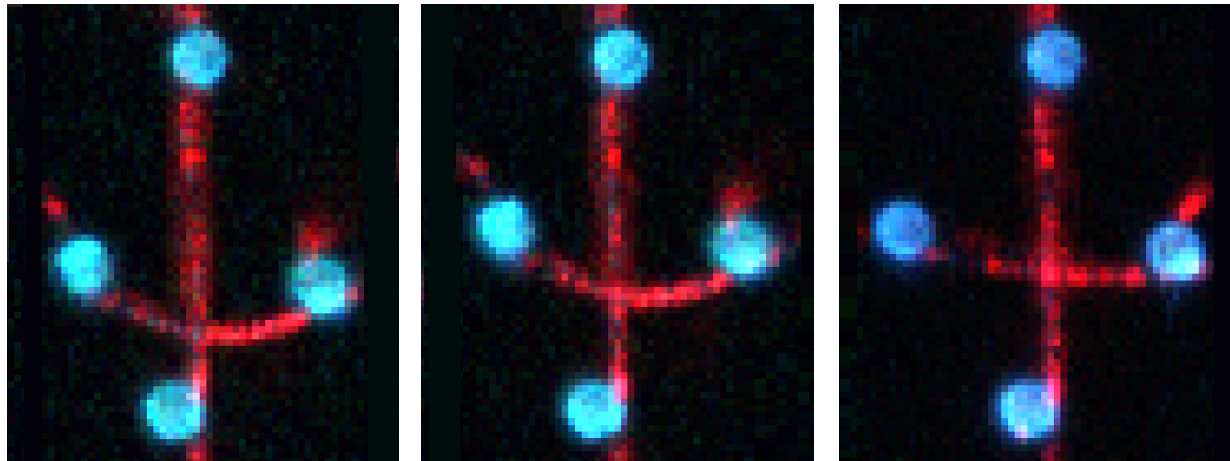
Figure 1 shows the interaction between two fluorescently labeled microtubules, as one microtubule is dragged across the other with a known force. In the middle and left snapshot, we can observe that the friction force between the filaments causes one microtubule to adopt a curved conformational structure. When we stop the movement of the microspheres the microtubule slowly relaxes (right).
The surface properties of these filaments, the electrostatic and ionic forces and specific interactions, can generate friction when two filaments interact. This friction can be measured using a device such as the C-Trap® configured for quadruple optical trapping.
Application note | Filament manipulation