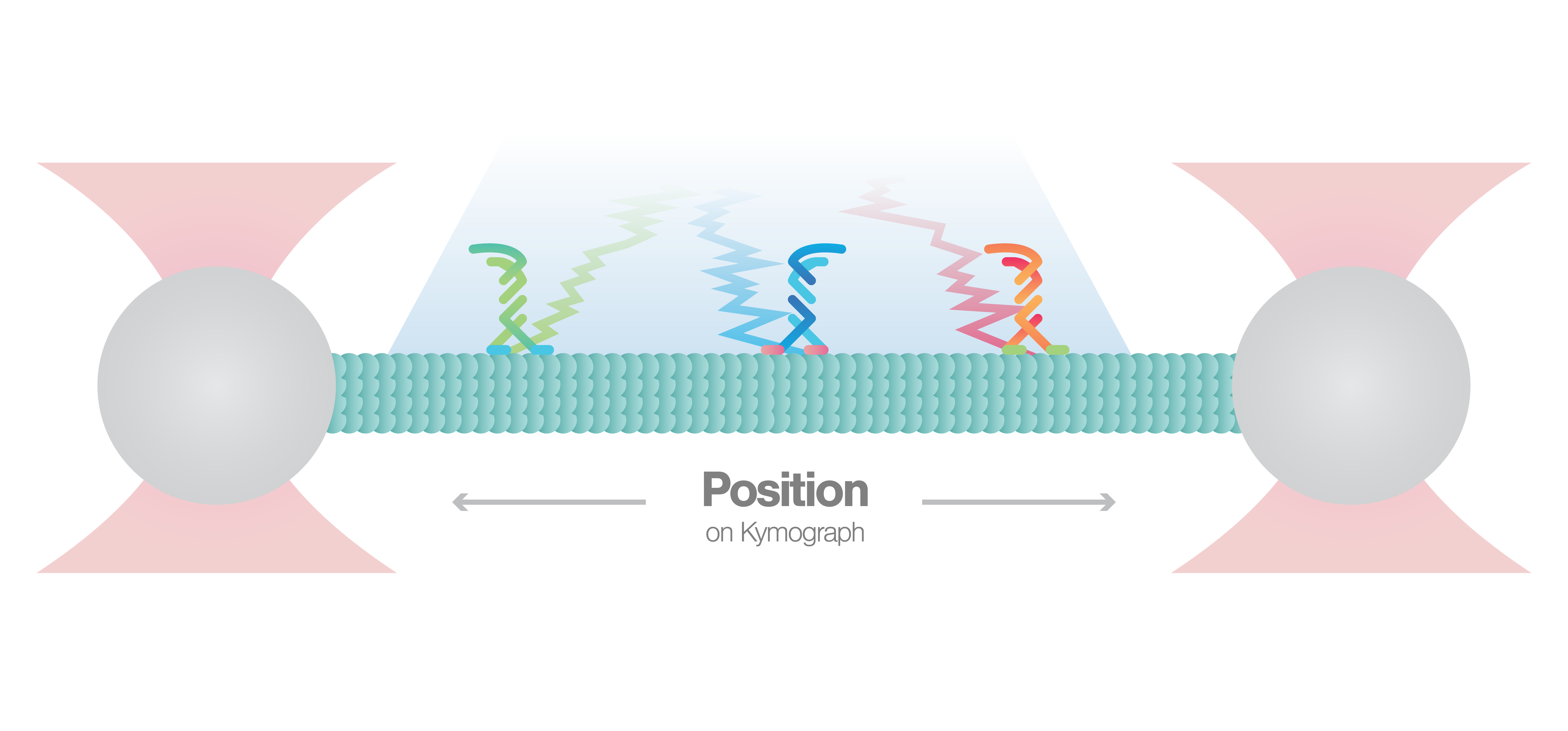
Single-molecule visualization of cytoskeletal motors in solution using confocal or STED
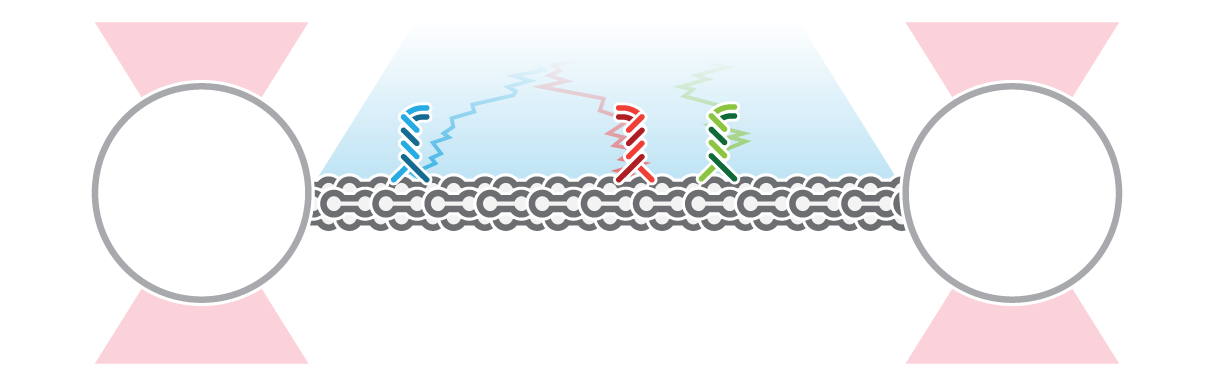
In this experiment a microtubule is tethered between two optically trapped beads with fluorescently-labeled motor proteins bound to it. We can uncover the binding location, (un)binding events and kinetics of the cytoskeletal motors by keeping the microtubule stretched and simultaneously scanning the motors over time with a confocal beam.
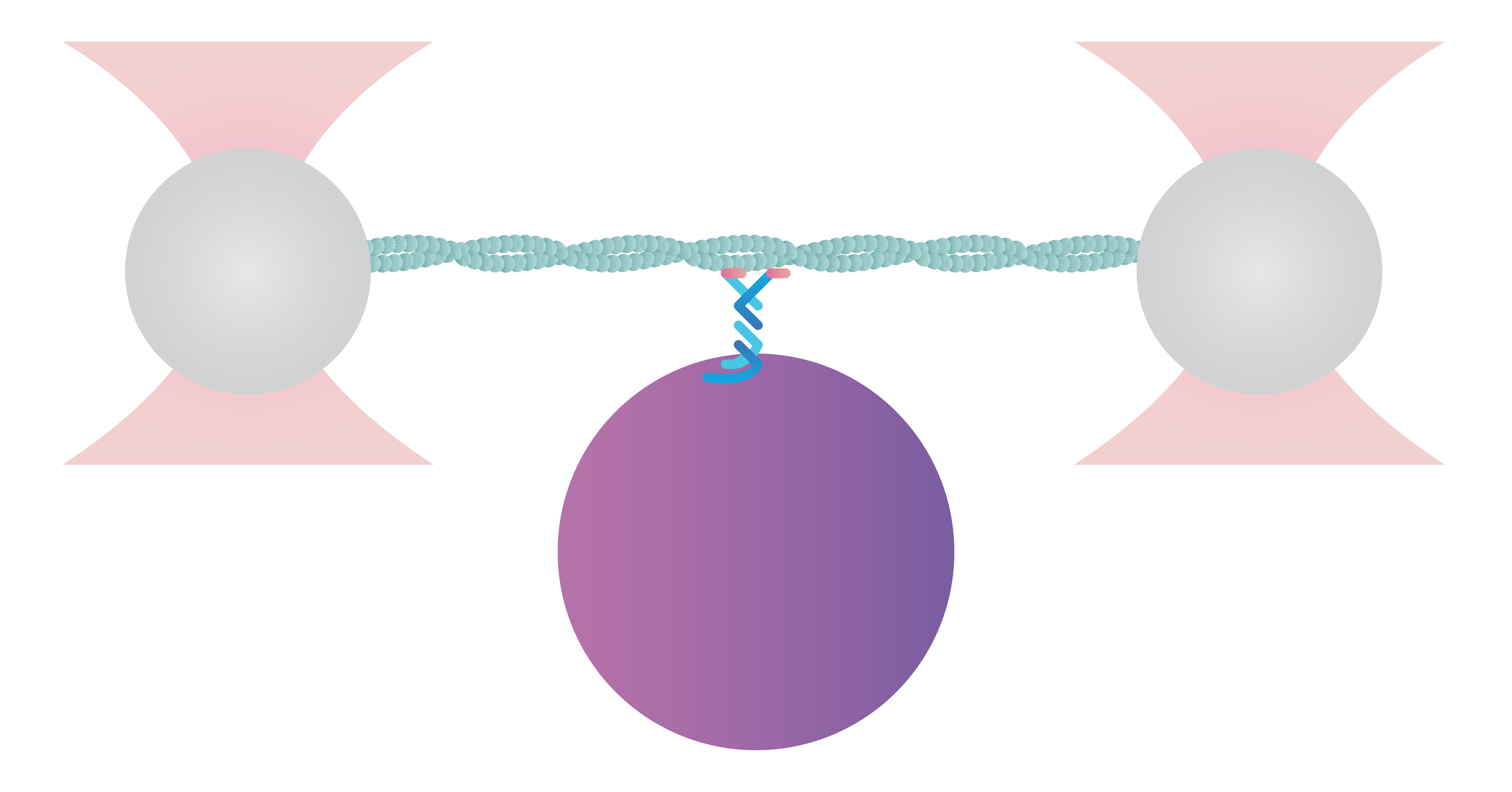
Investigation of motor protein stepping mechanics along cytoskeletal filaments
Here, a three-bead assay is utilized to study the stepping behavior of a molecular motor. Two optically-trapped beads hold actin tight and a third myosin-coated bead is immobilized on the glass surface of a flow cell. We can visualize the fluorescently-tagged actin filaments using correlated confocal microscopy and perform simultaneously distance measurements. This offers the possibility to track the activity of molecular motors along cytoskeletal proteins and determine their thermodynamic properties at the single molecule level.
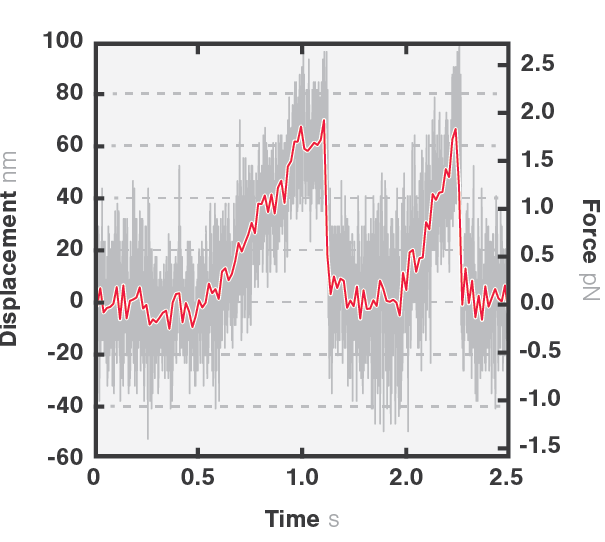
In this assay, we investigated the displacement of actin with respect to wild-type myosin-VI by measuring the correlated displacements of the two beads trapped in the dual optical trap. Figure 1 shows that myosin pulls the actin filament in a unidirectional manner with a measurable force.
The exact kinetics of the motor can be observed by measuring the relative translocation of the filament with respect to the motor and the related forces. From the resulting force–time traces, we can derive the speed and processivity of the motor.
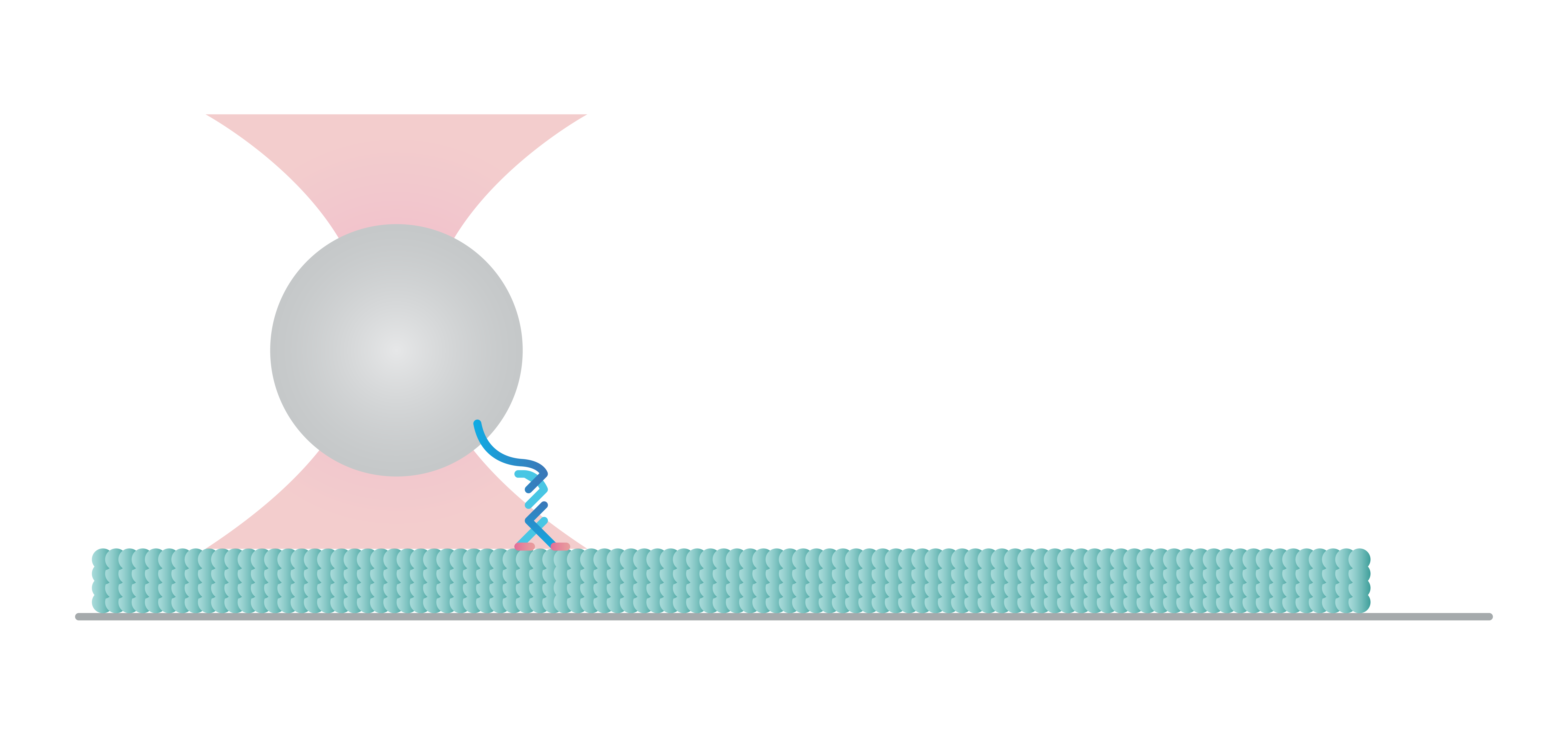
Stepping of filaments and motors at the surface
The C-Trap enables you to construct and test a variety of assays to understand the molecular mechanisms of motor-filament interactions and gain insights into their regulation. In this experiment we tethered a kinesin motor to a bead and placed the motor on a microtubule which we visualized with label-free IRM.
We can follow the motor’s activity in all 3 dimensions as it moves over the filament and measure the distance, velocity, and (stall-) force of a motor attached to a bead, also under the influence of other molecules and loads.
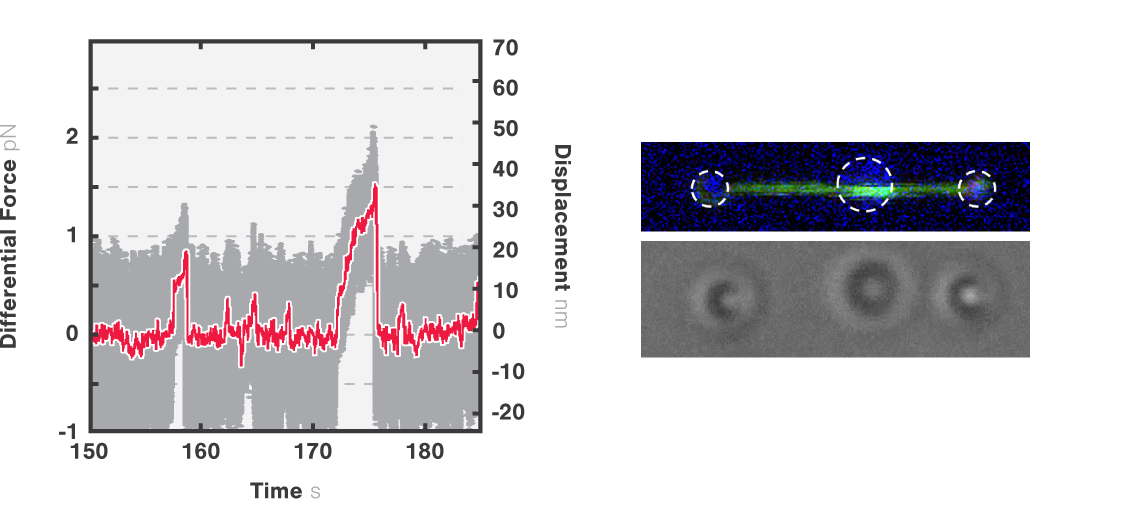
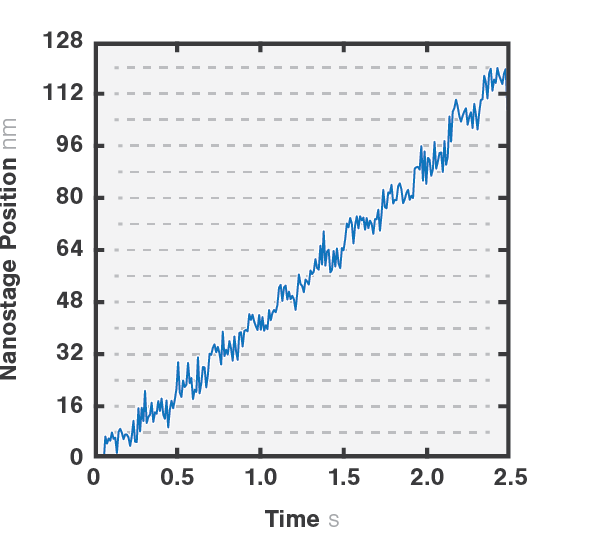
Figure 2 shows a single kinesin motor stepping along a microtubule. Steps, 8 nm in size, were collected with 2D feedback along the microtubule direction at stall force of 1 pN.
In Figure 3 we can observe 2 events during which the kinesin motor detaches from the microtubule at a force of approximately 2 pN.