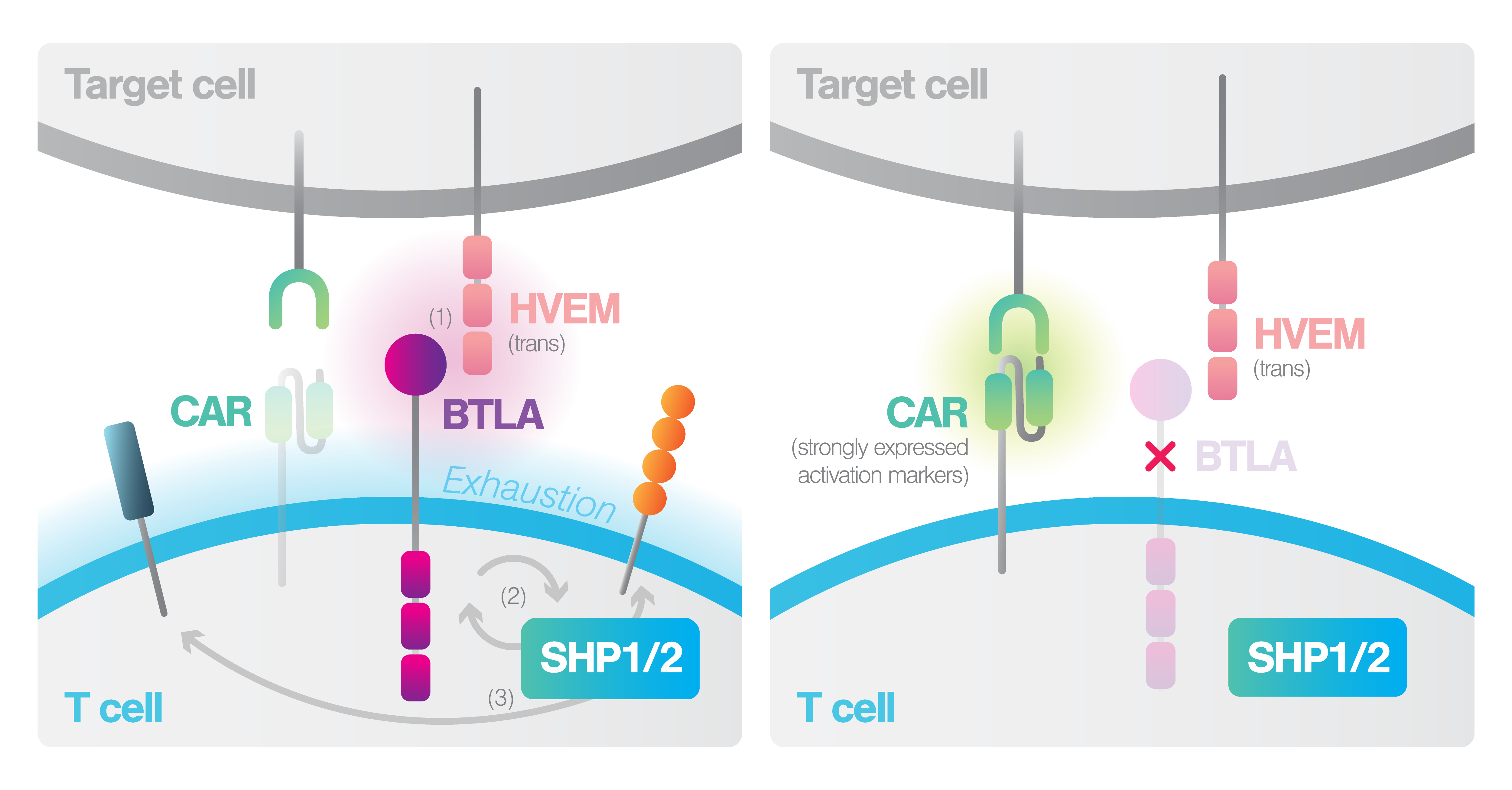
Recent advances in T cell-based immunotherapies, such as checkpoint blockade and adoptive cellular therapy, have revolutionized cancer treatment. However, the tumor microenvironment (TME) remains a significant barrier, limiting the effectiveness of these therapies through several immunosuppressive mechanisms. The TME often upregulates immune checkpoint molecules to inhibit T-cell activity. The BTLA-HVEM axis has emerged as a critical factor in T cell inhibition and exhaustion, particularly in Hodgkin lymphoma (HL), and in other cancers.
Cell avidity measurements uncovered the mechanism by which the BTLA-HVEM axis reduces cell-cell binding and were used to support rational CAR-T design, ensuring both potency and safety.
Breakthrough Nature Immunology publication reveals an avidity enhancement mechanism for CAR-T cells
Guruprasad et al., (2024) from the University of Pennsylvania demonstrated that enhancing cell-cell binding can help overcome immunosuppressive mechanisms deployed by the TME. In this study, researchers demonstrated that high BTLA expression on CAR-T cells correlates with poor clinical outcomes due to its interaction with HVEM on TME cells, leading to T cell inhibition. Moreover, BTLA expression was shown to be associated with poor outcome in patients with lymphoma treated with CD19 CAR T cells, approved by the FDA. By deleting BTLA in CAR-T cells, the study showed improved tumor control and persistence in both lymphoma and solid tumor models. This modification enhances CAR signaling , cell avidity and effector function, overcoming the immunosuppressive effects of the TME
Key findings:
Unique Mechanistic Insights: BTLA inhibits CAR-T cell function by recruiting SHP-1 and SHP-2, and its knockout enhances CAR– T binding and downstream signaling pathways.
Enhanced Potency: BTLA-deficient CAR-T cells showed greater cell-cell binding leading to improved expansion, cytokine secretion, and prolonged survival and effector function in vivo.
Sensitivity Revelations: cell-cell binding sensitivity was analyzed across a variety of BTLA expressing T-cells to confirm the likelihood of downstream activation. Removing BTLA from CAR-T cells targeting CD30 demonstrated enhanced tumor control in HL
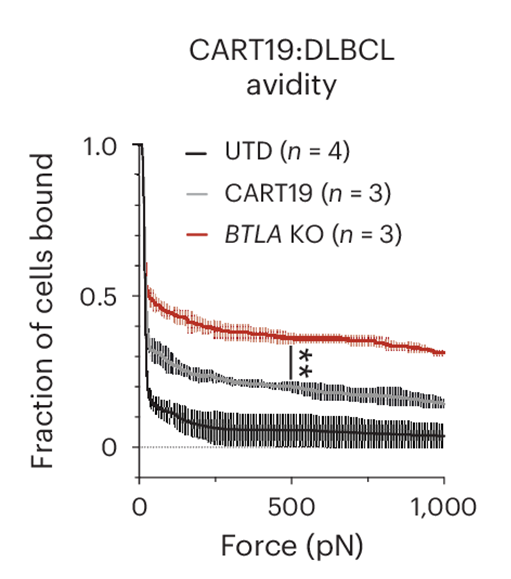
Crucially, the study utilized cell avidity measurements to assess the binding strength between CAR-T cells and tumor cells. By integrating LUMICKS’ z-Movi Cell Avidity Analyzer, the team demonstrated that BTLA-deficient CAR-T cells exhibited increased cell avidity, or binding strength, specifically with Nalm6 tumor targets when using CD19-targeting CAR-T cells. This increased binding avidity underscores the effectiveness of BTLA deletion in enhancing the CAR-T cell-tumor cell interaction, thereby enhancing potency.
From Hodgkin Lymphoma to solid tumors: The Impact of Cell Avidity to weaken the tumor microenvironment
The validation of CD19-targeting CAR-T cells in Nalm6 tumor models demonstrated that BTLA deletion increased cell avidity, resulting in stronger binding to tumor targets. These findings are particularly significant for Hodgkin lymphoma, where enhanced binding and reduced immunosuppressive effects in the TME could lead to substantial therapeutic improvements. This approach can be generalized to target other immune checkpoint molecules and other cancer-associated cell types in the TME, thereby improving therapeutic outcomes across various cancers. For example, a recent study published by Wehrli et al., (Clin. Cancer Res., 2024) developed a novel engineered CAR-T cell with enhanced avidity to target and bind strongly to both tumor cells and cancer-associated fibroblasts, weakening the TME and thus improving tumor cell cytolysis.
Employing cell avidity analysis deepens our understanding of the mechanisms and potency of novel CAR-T strategies. Ultimately, by fine-tuning immune checkpoint interactions and leveraging avidity metrics, we can develop more potent and durable treatments, reducing T cell exhaustion and enhancing antitumor responses.