Customer case study: Using cell avidity to explore the network of intercellular interactions of NK cells in the tumor microenvironment
Prof. Mark Lowdell
Professor of Cell & Tissue Therapy at University College London and Chief Scientific Officer of INmune Bio, Inc

About Prof. Lowdell and his research focus
The innate killing ability of NK cells serves as a compelling immunotherapeutic tool
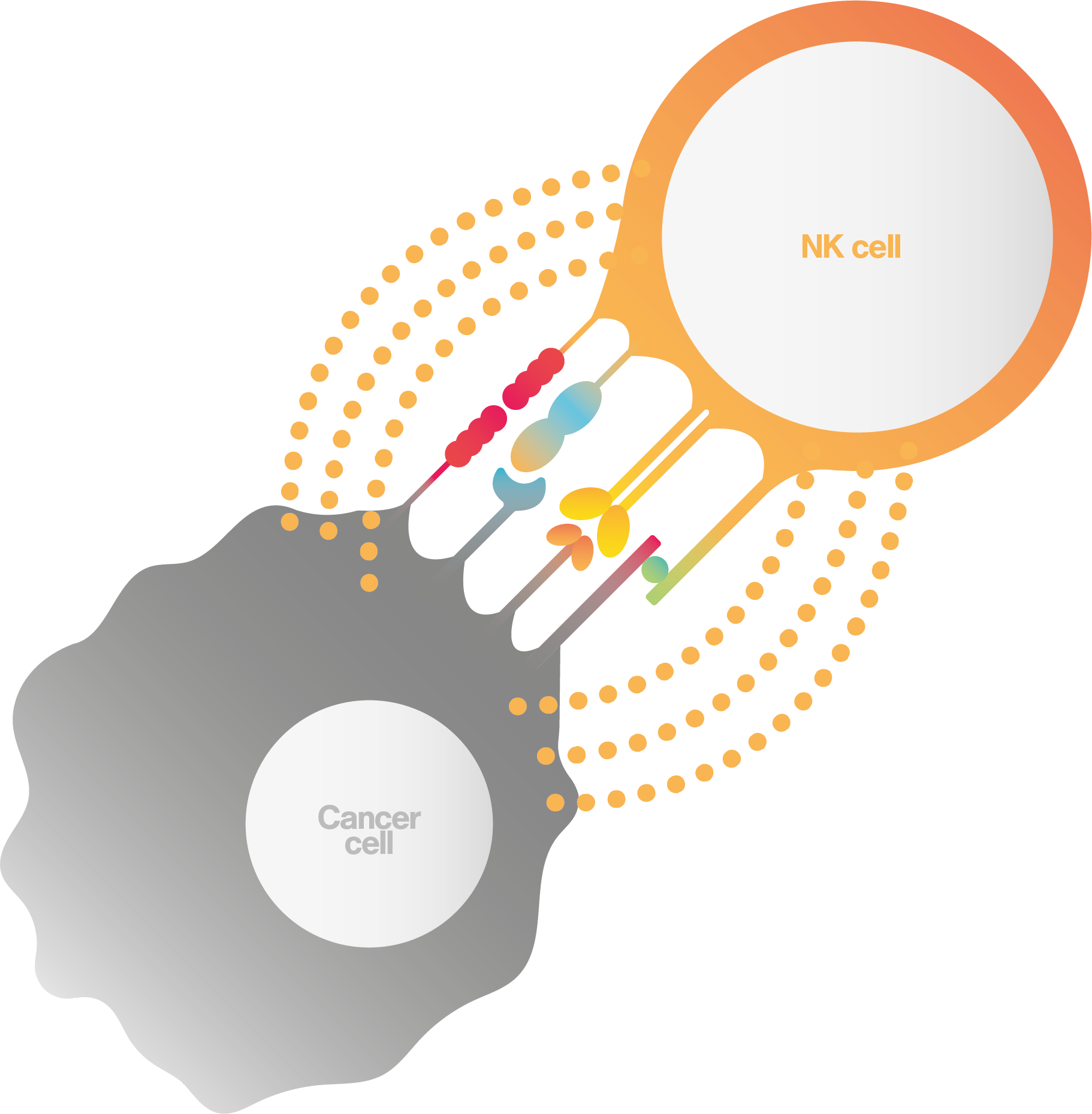
In the second mechanism, NK cells bind to the “death receptor” that sits on the surface of the cancer cell inducing signals that lead to cancer cell apoptosis.
NK cell failure is a major cause of cancer relapse
Overcoming NK cell failure with INKmune™
Exploring the Network of Cellular Interactions of NK Cells in the Tumor Microenvironment
Watch a snippet of Mark Lowdell’s webinar on finding the synapse with microscopy. If you would like to download the complete webinar, click here.
Watch now
Cell avidity analysis directly measures and quantifies the synaptic interaction
Using microscopical techniques, the team of prof. Mark Lowdell saw that the NK cells seemed to form a more stable synapse with the tumor cell when they were activated. Prof. Mark Lowdell and his team use the z-Movi® Cell Avidity Analyzer as a solution to directly measure and quantify the synaptic interaction.
With cell avidity measurements, the team can rapidly distinguish NK sensitive- from NK-resistant tumor cells by quantifying amount of NK cells bound to cancer cells. In their research studies, the binding between resting NK cells and NK sensitive- (K562) tumor cells depicted a higher avidity score, indicating the formation of a stronger immune synapse. On the other hand, the binding between NK cells and NK-resistant (SKOV3) tumor cells showed a significantly lower avidity score, indicating a much weaker binding compared to NK sensitive- (K562) tumor cells (Figure 1).
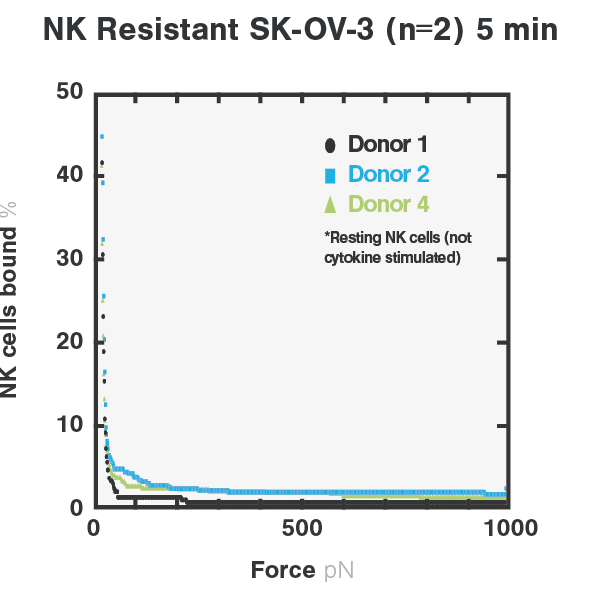
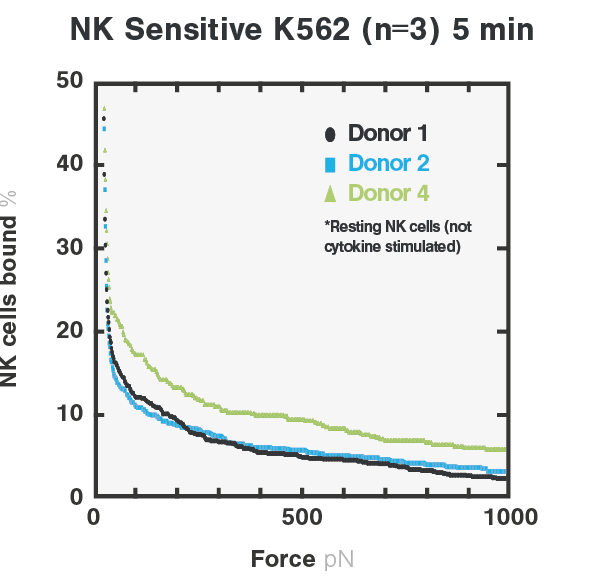
Figure 1. Avidity curves showing the percentage of target-bound resting NK cells upon increasing acoustic forces. Cell avidity measurements of NK cells were performed on NK-resistant SKOV3 tumor cells (left), or NK sensitive K562 tumor cells (right).
Cell avidity measurements depict stronger binding of INKmune-primed NK cells that eradicate residual disease
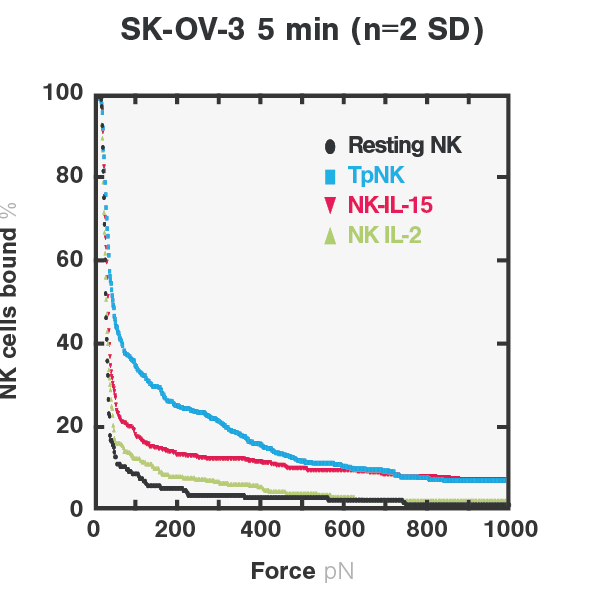
Figure 2. Cell avidity between resting-, INKmune-primed-, IL-2 primed-, IL-15 primed- NK cells and NK-resistant SKOV3 tumor cells.The avidity curves show the percentage of target-bound NK cells upon increasing acoustic forces. rForce indicates relative force.
Cell avidity technology provides key information to accelerate immunotherapy development
“The success of INKmune offers substantial clinical benefits, as INKmune-primed NK cells can become a more affordable immunotherapeutic option by in vivo activation of patient’s own autologous NK cells. It has become evident that beyond conventional assays, cell avidity measurements with the z-Movi® Cell Avidity Analyzer provide key information that can accelerate immunotherapy development by accurately predicting in vivo and clinical efficacy.”
Watch the full interview here
Watch the webinar!
In this webinar, Prof. Mark Lowdell how to use the z-Movi® Cell Avidity Analyzer to dissect the temporal nature of the synaptic formation between NK cell and tumor cell.


