Key takeaways
- Researchers have examined how format-tuning bispecific T cell engagers (BTEs) boosts therapeutic efficacy against clear cell renal cell carcinoma (ccRCC).
- Using a novel persistent multivalent T cell engager (PMTE) to enhance cell avidity and tumor targeting, they tackle challenges like low plasma half-life, poor tumor retention, and antigen escape.
- Optimizing cell interactions through avidity-driven design offers a pathway to more effective, durable cancer therapies and renewed hope for advanced ccRCC patients.
This is a summary of:
Ryan P O’Connell, Kevin Liaw, Nils Wellhausen, Christopher A Chuckran, Pratik S Bhojnagarwala, Devivasha Bordoloi, Daniel Park, Nicholas Shupin, Daniel Kulp, Carl H June & David Weiner (2024).
Format-tuning of in vivo-launched bispecific T cell engager enhances efficacy against renal cell carcinoma. Journal for immunotherapy of cancer, 12(6), e008733
Authors
Rogier Reijmers, PhD – Principal Scientist & Head of Collaborations
Andrea Candelli, PhD – CSO & Head of Marketing
Niels Tjoonk, MSc – Product Marketing Manager
The Problem: Design challenges of bispecific T cell engagers
First-generation bispecific T cell engagers (BTEs) have shown promise in preclinical models but often suffer from a short plasma half-life due to their small size and absence of an Fc domain. Continuous infusion solves this rapid clearance issue, but complicates clinical translation, patient burden and hampers overall treatment success. Additionally, limited tumor retention can lower local therapeutic concentrations and impede efficacy. Addressing these pharmacokinetic challenges is vital to enhancing the durability and impact of BTE therapies.
Compounding these issues, cancer cells can adapt and evade immune targeting through antigen heterogeneity and loss, threatening sustained responses. The immunosuppressive tumor microenvironment (TME) further hinders T cell function and promotes therapeutic resistance. To develop an effective strategy, any cell engager must overcome these barriers to achieve robust, consistent targeting and immune activation. This necessity is directly tackled by O’Connell et al. (JITC, 2024), whose work on format-tuning BTEs, targeting clear cell renal cell carcinoma (ccRCC), to improve cell avidity and retention represents a significant step forward, as discussed in this research brief.
The Study: Format-tuning of bispecific T cell engagers to enhance efficacy
Renal cell carcinoma, particularly its clear cell subtype (ccRCC), remains one of the most challenging cancers to treat, with advanced cases often resistant to traditional chemotherapies and targeted therapies. Despite progress with immunotherapies such as checkpoint inhibitors, many patients still exhibit limited or transient responses. BTEs may complement checkpoint inhibitors in expanding immunotherapy responsiveness in ccRCC and other cancers, providing their design challenges are met.
To address the challenges in optimizing BTEs for treating ccRCC, with this study, the researchers developed a persistent multivalent T cell engager (PMTE) that targets carbonic anhydrase IX (CA9 or CAIX) on ccRCC cells. Unlike traditional BTEs that utilize a dual single-chain variable fragment (scFv) design only, the PMTE incorporates an Fc domain alongside an additional binding site, providing bivalency for CA9. This multivalent design not only enhances cell avidity and tumor retention but also offers the benefit of Fc-mediated half-life extension, improving the therapeutic persistence of this cell engager molecule.
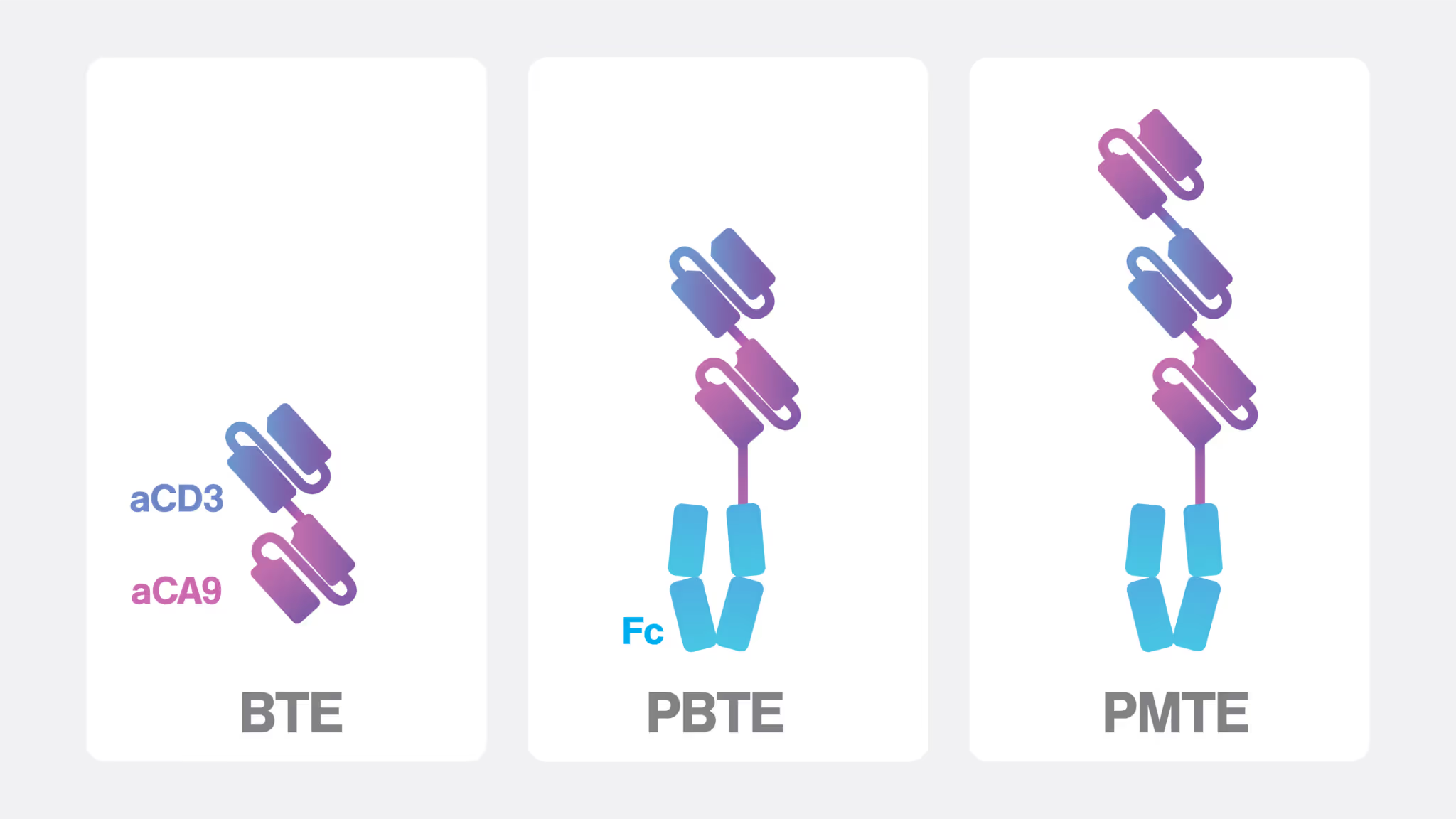
In several comparative experiments, the PMTE was evaluated against a first-generation BTE and a persistent BTE (PBTE) that features a single-chain Fc domain for extended circulation. While the PBTE exhibited improved pharmacokinetics relative to the conventional BTE, such as a 32-fold increased plasma half-life, its Fc domain contributed to a significantly reduced binding affinity and cell avidity, likely due to steric hindrance that limited target engagement.
In contrast, the PMTE not only restored but also exceeded the binding strength and avidity observed in the first-generation BTE, demonstrating superior performance in enhancing cell targeting and therapeutic efficacy.
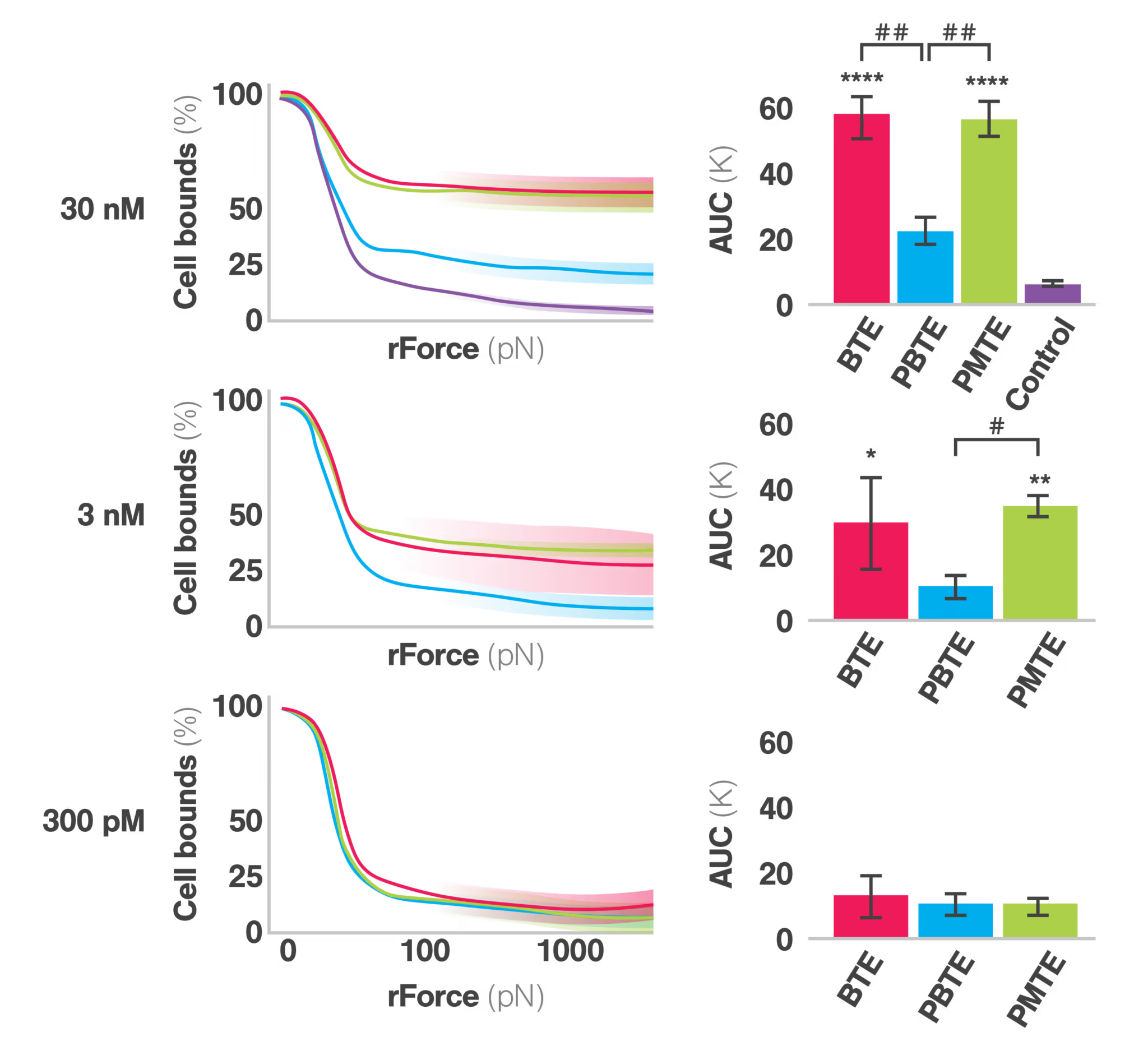
Characterizing bispecific T cell engager formats with cell avidity analysis
Measuring and optimizing cell avidity
Cell avidity, or the cumulative strength of binding interactions between the engager and target cells, is a proven method to reveal the mechanism of action and determining therapeutic efficacy. High cell avidity promotes sustained cell-cell interactions, enabling better immune synapse formation and enhanced T cell activation, as stated by the authors. To characterize cell avidity, the study employed the advanced LUMICKS technology, the z-Movi cell avidity analyzer, which uses ultrasonic force to measure the strength of interactions between immune cells and cancer cells at a single-cell resolution. This innovative approach provided quantitative mechanistic insights into how the different BTE formats influence synaptic strength and cellular engagement.
PMTE restores and enhances cell avidity
The PMTE format demonstrated significantly improved cell avidity compared to the PBTE format, which exhibited weaker synaptic strength and lower cytotoxic potency, even compared to the BTE configuration. The dual-targeting nature of the PMTE, by including an additional anti-tumor target (CA9) arm, allowed for stronger engagement with CA9-expressing cells, leading to more stable interactions and improved T cell activation.
This increase in cell avidity translated into a marked enhancement in tumor cell killing in vitro and in vivo, and superior pharmacokinetic behavior because of the Fc domain inclusion, with better tumor retention and distribution.
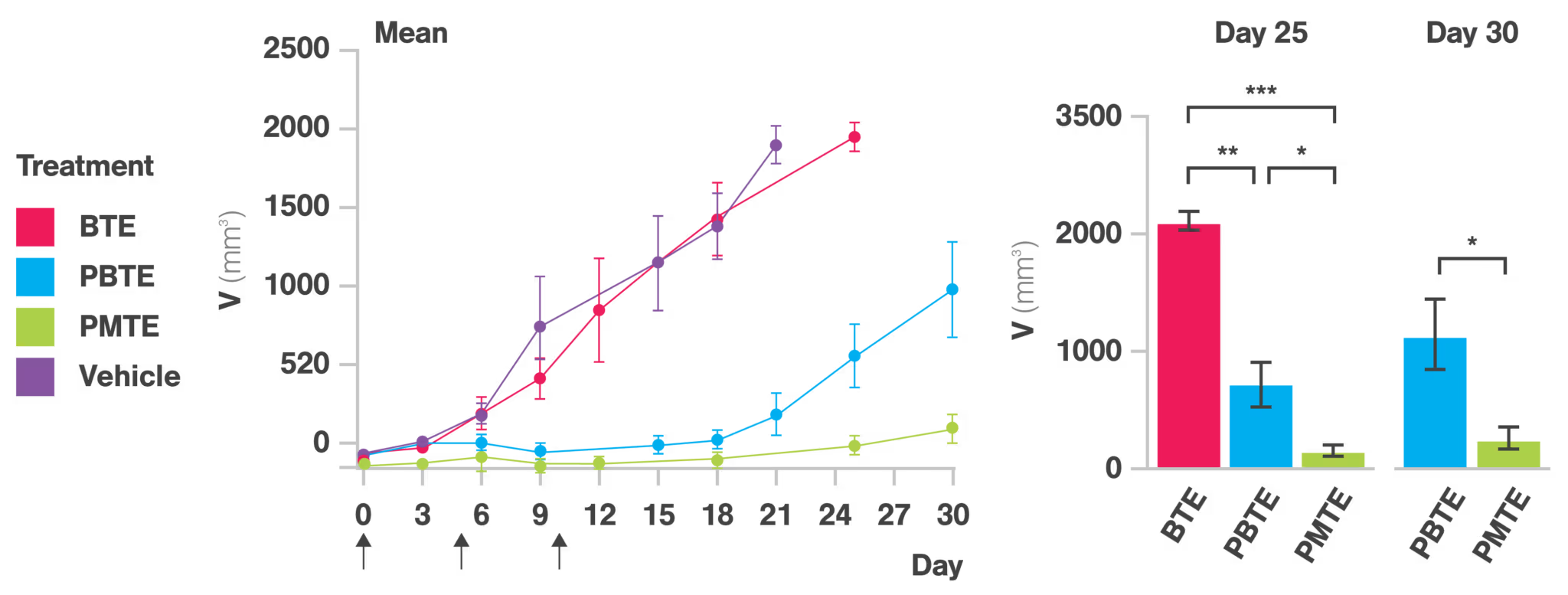
The implications: Balancing pharmacokinetic benefits with cell avidity optimization for cell engager success
Superior tumor cell killing
In vitro cytotoxicity assays highlighted the potent killing ability of the PMTE, its improved cell avidity, and multivalency allowing for stronger and more sustained interactions with tumor cells. Importantly, this effect was observed across multiple ccRCC cell lines, reinforcing the broad applicability of this format, perhaps even extending to many different tumor types, making this a general formatting approach for BTEs.
Pharmacokinetic advantages
The PMTE demonstrated a markedly prolonged plasma half-life compared to the conventional BTE, thanks to the pharmacokinetic advantages conferred by its Fc domain. Notably, unlike the PBTE, which experienced reduced tumor penetration due to impaired cell avidity, the PMTE achieved superior tumor retention and distribution. This highlights the critical need to balance pharmacokinetic enhancements with cell avidity optimization to maximize therapeutic efficacy. Interestingly, despite a decrease in cell avidity, the PBTE’s inclusion of an Fc domain led to improved tumor control in vivo, emphasizing the significant impact of combining extended half-life and increased cell avidity on therapeutic outcomes.
The Broader Context: how antibody engineering can boost therapy success
Antibody-based therapeutics have become pivotal in modern medicine, representing nearly one-fifth of new FDA drug approvals each year. This trend underscores their growing market significance and expanding role in treating complex diseases, particularly cancer. Despite their success, challenges such as limited therapeutic responses (poor tumor penetration or hostile microenvironment), drug resistance, and side effects remain critical hurdles. However, ongoing innovations in antibody engineering offer promising strategies to overcome these limitations and propel the next generation of antibody-based therapies.
Diversifying the antibody landscape
While IgG monoclonal antibodies remain the cornerstone of antibody therapeutics, recent advancements are expanding the field’s horizons. Emerging platforms have facilitated the development of bispecific antibodies, which can simultaneously bind to two different targets, offering enhanced specificity and functionality. Additionally, antibody-drug conjugates are gaining traction by enabling targeted delivery of cytotoxic agents, thereby minimizing off-target effects. Novel antibody fragments, such as nanobodies and antibody fusion proteins, further broaden the toolkit available to researchers, providing smaller, highly stable, and versatile alternatives for therapeutic applications.
Advancing the antibody field with novel technologies
The future of antibody-based therapies lies in the continuous refinement of molecular engineering techniques, the expansion of antibody formats, and the strategic integration of advanced technologies, including cell avidity analysis. By addressing current challenges and unlocking new therapeutic possibilities, antibody engineering is poised to revolutionize the treatment landscape, offering more effective, personalized, and targeted solutions for complex diseases. The PMTE format highlights one such an example of successful engineering and the potential for further advancements in bispecific and multispecific antibody therapeutics. By tuning binding valency, Fc domain modifications, and cell avidity, future therapies can be tailored to specific tumor antigens and immune cell targets, enhancing efficacy across diverse cancer types. Most importantly, this study demonstrated that combining cell avidity measurements with classical assays revealed the mechanism of action, which was unknow thusfar, making rational design choices easier to understand and empowers better-informed decision-making going into the clinic.
Overcoming tumor microënvironmental barriers
The increased cell avidity and targeting precision offered by the PMTE may also help circumvent immune suppression within the TME. By driving stronger immune synapses and focused activation, PMTEs can potentially overcome the suppressive signals and physical barriers that limit the efficacy of many immune therapies, which could be explorations for the future.
Conclusions
This study by O’Connell et al. marks a fascinating example of how format-tuning and cell avidity optimization can transform BTE therapies specifically, and antibody therapy in general, for challenging cancers like ccRCC. By addressing key limitations of earlier designs, the PMTE format demonstrates a clear path forward in enhancing therapeutic efficacy, providing a robust model for future innovations in antibody-based precision immunotherapy.
Notable Researchers: Renowned scientists utilizing Cell Avidity for developing Cell Engager therapies
1. Dr. Marcela Maus, PhD, Associate Professor of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, US
Dr. Marcela Maus is Associate Professor of Medicine at Harvard Medical School and the Director of the Cellular Immunotherapy Program at Massachusetts General Hospital. Dr. Marcela Maus is actively involved in developing and improving CAR T cell therapies, including work on Cell Engagers. Her research focuses on using genetic engineering to redirect T cells to target and kill tumor cells while sparing healthy tissues. This involves designing molecular receptors to target T cells to both liquid and solid tumors.

Cell Avidity measurements to understand and enhance CAR-T/tumor interactions in acute myeloid leukemia
CAR-T therapy can be a highly effective treatment for certain blood cancers such as acute myeloid leukemia (AML), but the mechanisms underlying productive CAR-T cell/tumor interactions are only beginning to be understood. Recent work has demonstrated that productive interactions can be influenced by the density of the target antigen on the tumor cell, stability of the CAR molecule itself, and additional antigen-independent and antigen-regulated interactions mediated by adhesion molecules. The sum total of the T-cell interaction with an antigen-bearing target cell is known as avidity. Watch this webinar from Marcela Maus to learn about novel CARs and the use of avidity measurements to understand and enhance productive CAR-T cell interactions with both liquid and solid tumors.