Customer case study: Increased cell avidity through CD38 co-receptor drives enhanced
BCMA CAR T-cell sensitivity and persistence
Dr. Maria Themeli
Group Leader of Hematology Department at
Amsterdam UMC Cancer Center Amsterdam

About Dr. Themeli and her research focus
Dr. Maria Themeli, Group Leader at the Hematology Department of Amsterdam UMC (VUMC), Cancer Center Amsterdam (CCA), has extensive experience in chimeric antigen receptor-engineered T cell (CAR T) design and development. During her post-doctoral research in the lab of Dr. Michel Sadelain at Memorial Sloan Kettering Cancer Center, she reported the use of induced pluripotent stem cells (iPSC) as a broadly applicable source of therapeutic CAR T cells. Currently, her research group’s focus lies in the development of innovative, off-the-shelf CAR T cell therapeutics against hematological malignancies, especially multiple myeloma and leukemia. Their efforts mainly aim to improve safety, efficacy and persistence of the existing single-targeted CAR T-cell approaches by implementing a multiplexed strategy.
Find out how collaboration with LUMICKS helps the research team to understand the mechanistic insights for rational design of dual-targeting CAR T cells.
The distinct ability of cell avidity technology to provide critical insights into engineered cells
“The z-Movi platform’s distinct ability to measure and quantify avidity provides a powerful tool for immuno-oncologists. The instrument provided an essential piece of evidence in our investigations, giving us critical insight into how these engineered cells work and what is ultimately responsible for their changes in behavior with this new dual-targeting strategy. Understanding the mechanism of action is fundamentally important for any effective drug development study.”

Tumor escape due to low antigen density and limited efficiency in single-targeting BCMA CAR
B-cell maturation antigen (BCMA) is a potential target antigen in multiple myeloma due to its highly selective expression in malignant plasma cells. BCMA targeted CAR T-cell therapy has been proven to be effective in patients with multiple myeloma. Despite that complete remission level reaching around 60-86% (Ali et al, Blood 2016; Zhao et al. J Hematol Oncol. 2018; Raje et al. NEJM 2019), a subset of patients still experience tumor relapse. One of the major suggested mechanisms for CAR T cell failure and tumor relapse in patients is significantly reduced antigen expression level, lowering the success of antigen target recognition and target tumor elimination. Other contributing factors are the lack of in vivo T-cell expansion, limited persistence and intrinsic dysfunction (exhaustion) of CAR T cells. Altogether, low antigen density on the tumor and insufficient efficiency of single-targeting BCMA CAR T-cells signify the need for a more effective approach.
Watch a snippet of Maria Themeli’s webinar on factors
that contribute to the CAR T-cell failure.
Dual-targeting with CD38 co-receptor improves BCMA CAR efficacy against multiple myeloma
To improve sensitivity and persistence of BCMA CAR T cells, the research team led by Dr. Themeli developed dual-targeting CAR T-cells, producing both anti-BCMA CAR and a chimeric costimulatory receptor (CCR) against CD38. CD38 becomes an attractive target due to its high expression in a variety of haematological malignancies. With the dual-targeting approach, the team aims to enhance BCMA CAR T-cell avidity to target cells, resulting in better cytotoxic potential against myeloma cells. To drive a stronger therapeutic immune response, they further incorporated two costimulatory signals, CD28 and 4-1BB, into the dual-targeting approach. The CD28 costimulatory domain helps to elicit faster and stronger cytotoxic capacity, especially in response to tumor cells with low antigen density, and the 41BB costimulatory domain helps to enhance in vivo persistence and drive higher expansion rate of CAR T cells.
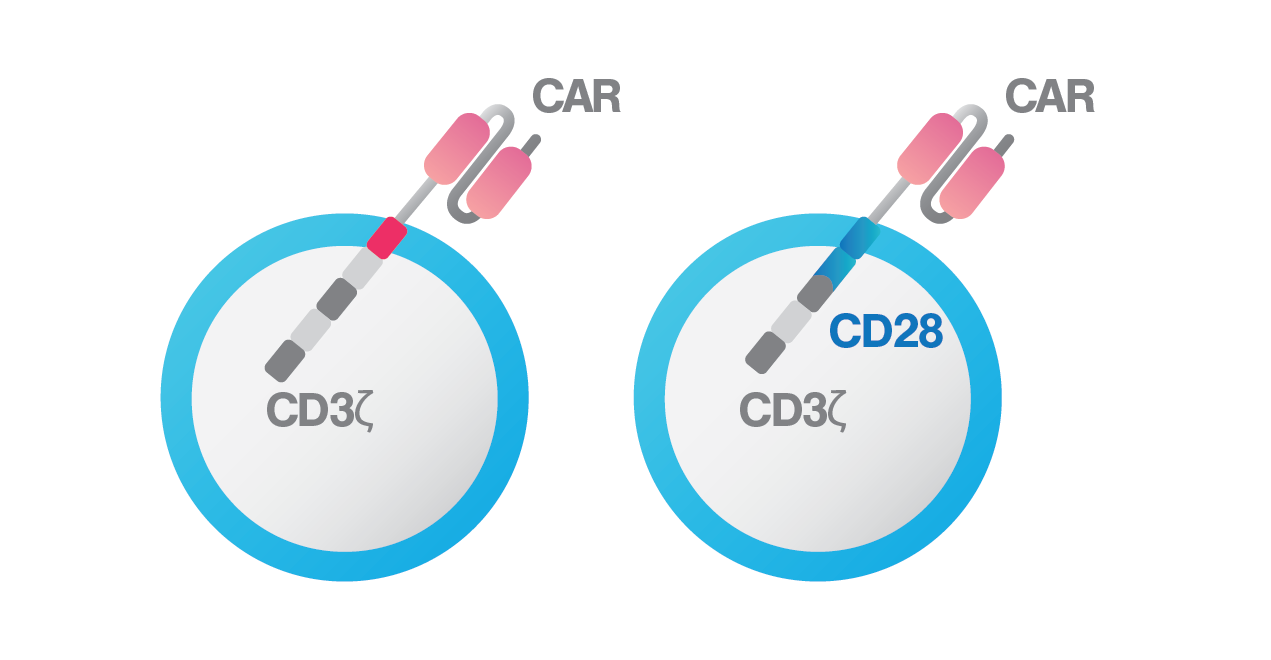
BCMA-3ζ CAR on the left and BCMA-28ζ on the right
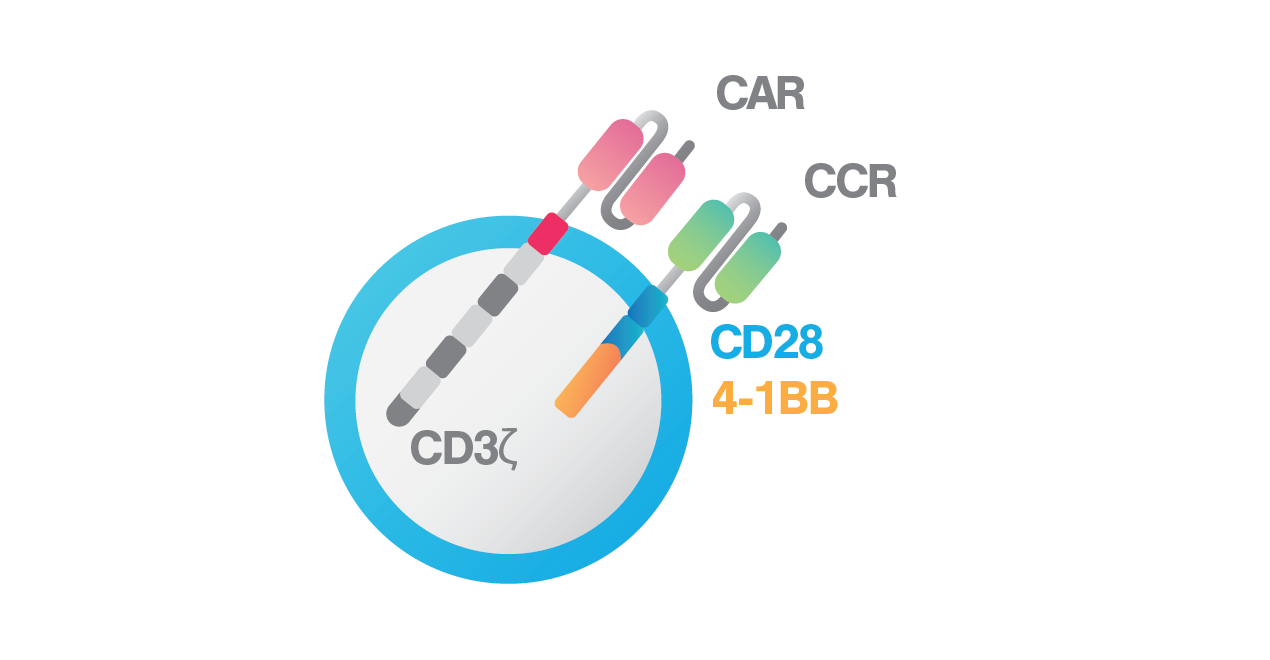
BCMA-ζ + CD38-28BB
Increased cell avidity due to multivalent engagement through BCMA CAR and CD38 CCR drives enhanced sensitivity
Tumor escape through target antigen downregulation is one of the known mechanisms that leads to patient relapse. To counteract this effect, the researchers set out to investigate if they could increase the cell avidity of BCMA CAR T-cells against target cells expressing low levels of BCMA by co-transfecting the T-cells with a CD38 targeting CCR. Indeed, cell avidity of BCMA-CD38 CAR/CCR T-cells for the target cells, as measured using the z-Movi® Cell Avidity Analyzer, was increased compared to the parental BCMA targeting CAR T-cells (Fig. 1a & 1b). In agreement with these results, the cells showed increased in vitro cytotoxicity against their target cells (Fig. 1c), indicating that enhancing cell avidity by co-targeting CD38 using a CCR enhances BCMA CAR T-cell functionality.
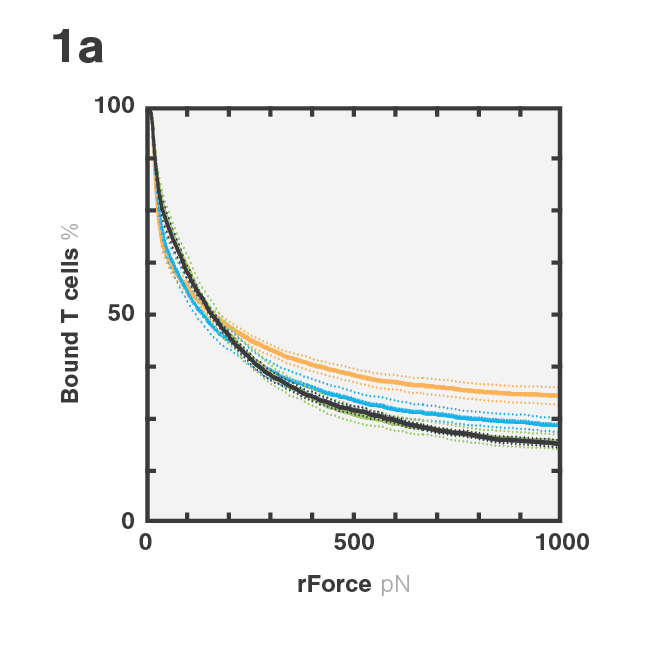
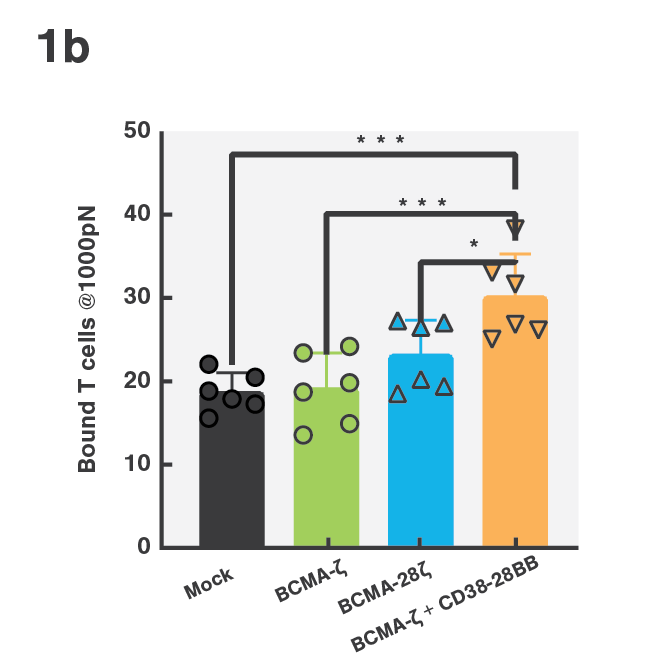
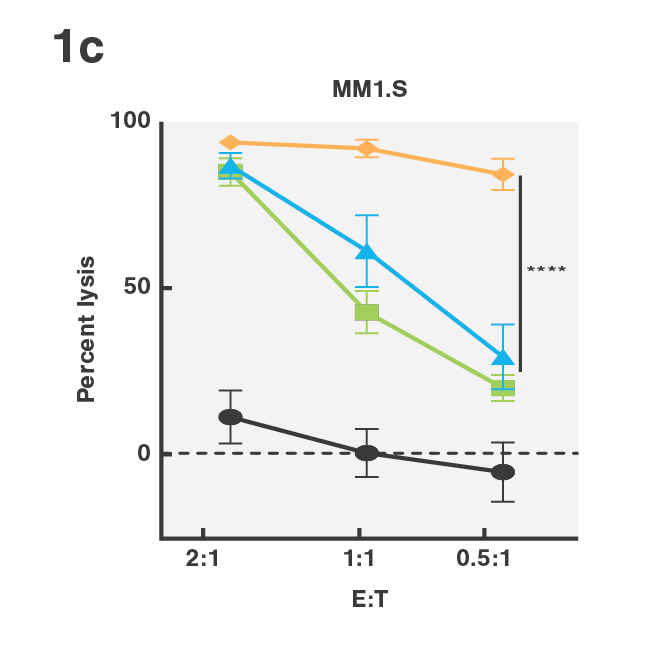
Figure 1 (A) Avidity curve and (B) bar graphs showing the average percentage of bound T cells at indicated force applied for indicated constructs against MM1.S cells. (A) Cells transduced with indicated constructs were co-incubated with MM1.S cells at indicated E:T ratios. (A,B) *P<0.05, ***P<0.001, Mean ±SEM from 6 measurements, p-values calculated using a paired t test. (C) ****P < 0.0001. Two-way ANOVA and subsequent multiple comparisons, corrected by Tukey test.
The power of cell avidity as a functional biomarker for improved CAR T design in preventing tumor relapse
This work, published recently in Science Translational Medicine (Katsarou et al, Science Translational Medicine 2021) demonstrated that assessing cell avidity with the z-Movi Cell Avidity Analyzer helped researchers to quickly identify that co-targeting induced higher cell avidity, even against antigen-low tumor variants. The data proves that adding the CCR increases the valency at the cell-cell interface, essentially giving the cells an extra ‘hook’ to hold on to their target, leading to enhanced avidity and higher cytotoxic potential of the cells. These results demonstrate how cell avidity analysis provides a deeper understanding of the mechanism and binding requirements behind multivalent binding, and that cell avidity is indeed a crucial biomarker for immuno-oncology. The application of this multiplexed strategy could improve clinical outcomes, reducing the number of patients relapsing due to tumor escape.
Download the
Research Highlight!
Read the full customer case on how cell avidity measurements with the z-Movi helped the research team to understand the mechanistic insights for rational design of dual-targeting CAR T cells.
Watch the
webinar!
In this webinar, Dr. Maria Themeli presented her study on how to overcome low antigen density and improve CAR T-cell persistence with multi-targeting and co-stimulation through increased synaptic avidity.
Read the
press release!
Find out how LUMICKS partook in the study published in Science Translational Medicine with cell avidity as a functional biomarker for improved CAR-T design in preventing tumor escape.
Learn more about the z-Movi®
Cell Avidity Analyzer
The z-Movi is a unique instrument that measures the avidity between immune cells and their targets, enabling you to identify the most potent immunotherapeutic effector cells.
Download the webinar & research highlight today:



