Avidity tuning led to a 100% response rate in lymphoma
Commercial CAR-T therapies still suffer from severe limitations, as majority of patients fail to achieve complete response and ultimately relapse.
Watch the new Webinar by Prof. Marco Ruella at UPenn to discover how his team have adopted a novel CAR-T avidity screening method to improve safety and exhaustion profile, leading to 100% clinical response in a phase I trial.
“To better understand the functional characteristics and mechanisms that support the increased anti-tumor efficacy of h1218-CART19 versus FMC63-CART19…we first quantified the cellular avidity of FMC63- CART19 and h1218-CART19 using the z-Movi platform (LUMICKS)”
Zhang et al., Molecular Cancer, 2023
Key takeaways:
- Explore the groundbreaking approach of selecting the right CAR for cell therapy application. Learn how an innovative CAR binder leads to enhanced clinical response compared to currently available commercial CAR. What makes this different from conventional CAR T cell therapies?
- Discover how tuning the fine balance between T cell engagement and exhaustion in CAR T cells leads to a more durable anti-tumor response and superior clinical outcome.
- Uncover the innovative strategy used to reduce Activation-Induced Cell Death (AICD) in CAR T cells, a key challenge in providing durable and complete response for patients.
Cell Avidity Unravels the Downfall of the APRIL CAR: Insights from a Failed Clinical Trial
Latest publication by University College London (UCL)
A recent JITC paper “Limited efficacy of APRIL CAR in patients with multiple myeloma indicate challenges in the use of natural ligands for CAR T cell therapy” published by the research team of Dr. Lydia Lee unveils the potential of cell avidity measurements in averting the failure of the APRIL-CAR clinical trial in a retrospective study.
How Dr. Lee described the z-Movi technology:
“In this unique exploration of the reasons underpinning suboptimal clinical responses in the AUTO2 trial, the z-Movi allowed us to establish poor tumor binding (or avidity) by cell bound APRIL compared to competitor CARs as a likely explanation. Only with such studies will the field acquire a greater understanding of the key preclinical readouts that can predict efficacy in patients.”
Summary:
- APRIL CAR was well tolerated in patients; however, the clinical responses were poor leading to 9 out 11 patient deaths
- APRIL CAR demonstrated significantly reduced cell avidity to BCMA expressing target tumor cells compared to other FDA approved BCMA CAR-T cells
- While traditional in vitro and in vivo (mouse) assays failed to identify the poor clinical efficacy of APRIL CAR, cell avidity successfully pinpointed the issue
Learn more about how cell avidity predicted the poor clinical outcome from the APRIL CAR trial and how to accelerate your path to successful IND filings
NEW: Watch Dr Lee’s interview to learn how avidity can improve the prediction of clinical outcomes
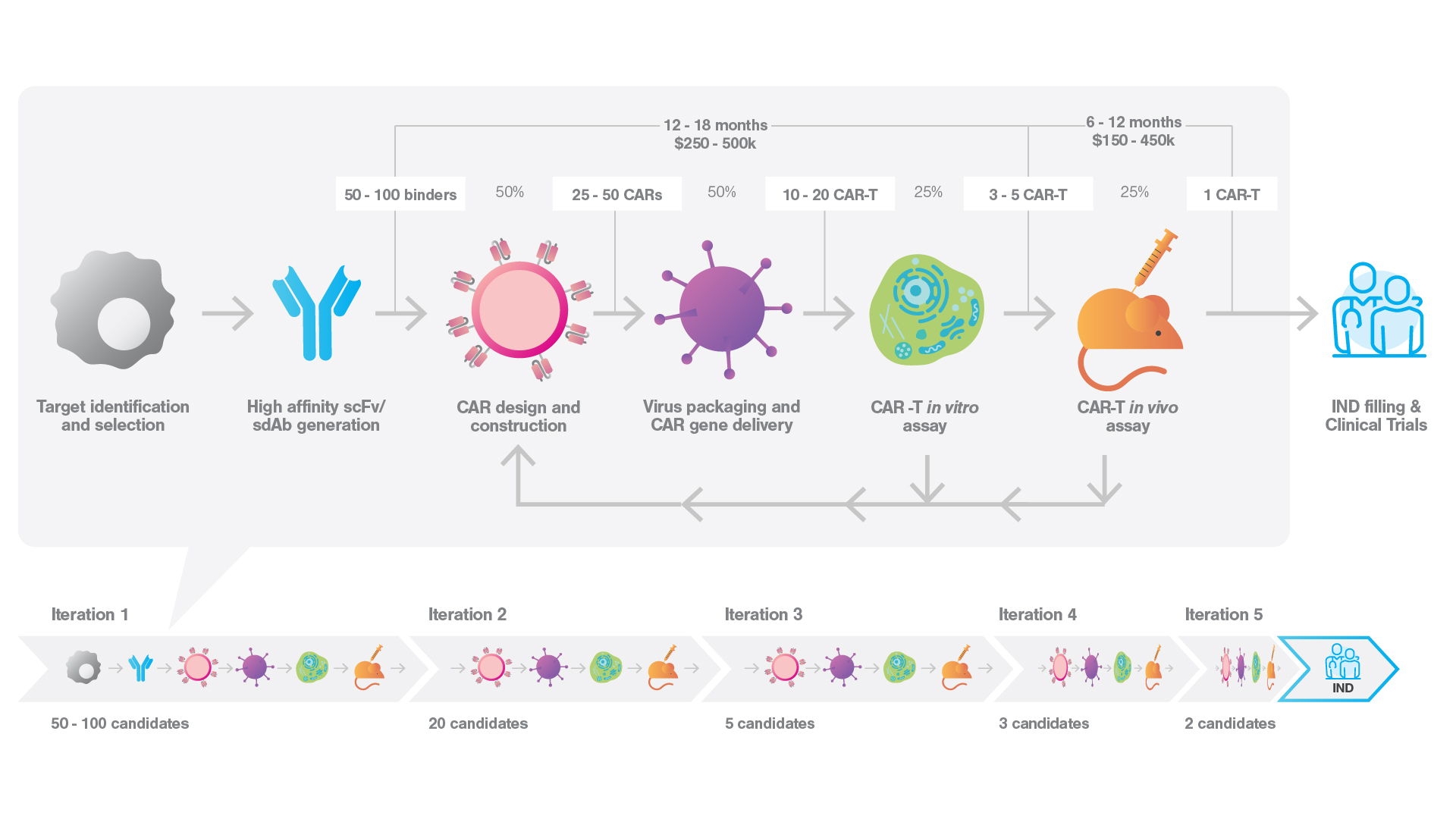
From concept to clinic: unravelling the iterative, time-consuming, and expensive process of cell therapy drug development
A staggering 83% of CAR-T lead candidates fail post-preclinical development, a trend that may worsen as we tackle more complex target. This not only prolongs the highly iterative development process, but also significantly inflates costs with a high failure rate in clinical trails.
We’ve been heavily dependent on the same in vitro endpoint assays, such as cytokine secretion and killing, during preclinical development. While these traditional assays can produce valuable data on a molecular level, they fall short in providing understanding of cellular interaction. This is a critical, yet often overlooked, piece of information.
Thus, we’ve historically had to make an educated guess when selecting a lead candidate, based on the limited information at hand. However, recent advancements have started to change this scenario…
Leveraging cell avidity measurements to accelerate your path to successful IND fillings
Cell avidity measurements produce a real-time readout on the initiating event of cellular killing, providing an improved prediction of in vivo efficacy compared to current in vitro assays (Larson et al. 2022, Nature, Chockley et al. 2023, Nature Biotechnology, Leick et al. 2022, Cancer Cell), which produce highly variable readouts.
Incorporating cell avidity measurements in the early stages of cell therapy development helps to streamline the process, reducing iteration cycles, inconclusive results and in vivo failures. This facilitates quicker identification of superior candidates, accelerates IND filings, and boosts R&D efficiency, saving both time and costs.