Accelerating Preclinical Development of Cell Engagers with Cell Avidity
Cell Engagers (CE) or bispecific antibodies are molecules that can steer immune cells, such as T cells, NK cells, and macrophages, towards cancer cells.
Did you know that more than 90% of immuno-oncology drugs (including CEs) entering phase I clinical trials fail to meet expectations due to poor efficacy and/or severe side effects?
Conventional in vitro methods used for characterizing Cell Engagers during preclinical development often produce inconclusive outcomes. This drawback leads to the selection of suboptimal candidates for in vivo testing and, in some cases, even for Investigational New Drug (IND) filings. This limitation significantly hampers progress, leaving drug developers vulnerable to failures at crucial stages of their journey.
Learn more about how Cell Avidity can accelerate your preclinical workflows and path to successful IND filings
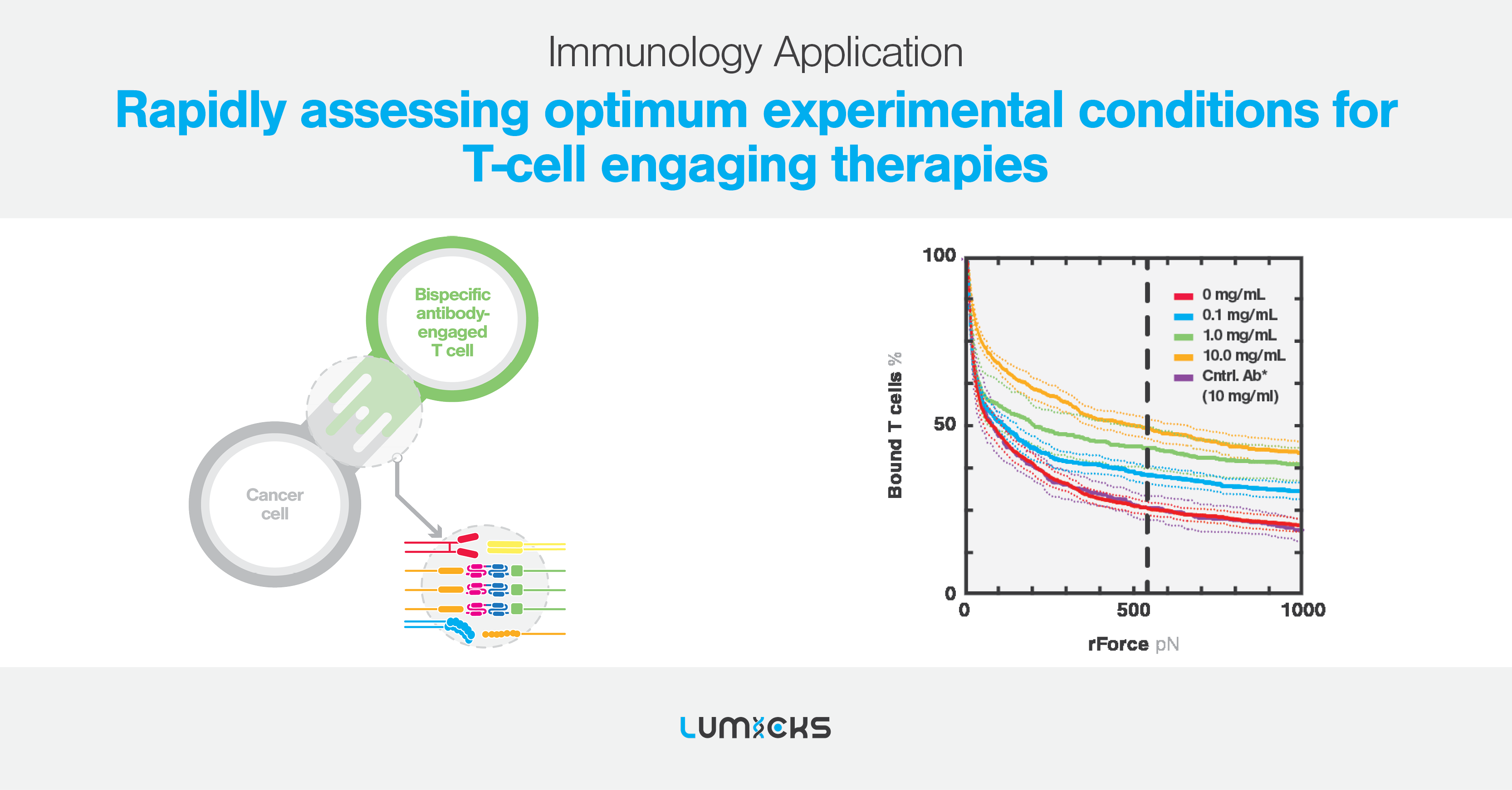
Improve decision making with high throughput avidity readouts
The incorporation of measuring avidity at high throughput at the outset of preclinical development brings forth an invaluable additional data point that was previously absent.
Findings derived from measuring the avidity of T-cells, in the presence of CEs, reveal unique information on the functional binding between effector and tumor cells. This provides researchers with more confidence in lead candidate selection by providing better differentiation between lead CEs compared to traditional in vitro assays
Our solutions
Cell avidity as a service
Cell Avidity analysis can be performed at high throughput using our services platform, enabling the screening of hundreds of Cell Engagers to rapidly find the most potent Cell Engager for in vivo testing.
Dr. Rogier Reijmers
Principal Scientist at LUMICKS



