This is a summary of:
Arellano-Ballestero, H., Zubiak, A., Dally, C., Orchard, K., Alrubayyi, A., Charalambous, X., Michael, M., Torrance, R., Eales, T., Das, K., Tran, M. G. B., Sabry, M., Peppa, D., & Lowdell, M. W. (2024).
Proteomic and phenotypic characteristics of memory-like natural killer cells for cancer immunotherapy.
Journal for immunotherapy of cancer, 12(7), e008717.
Authors
Andrea Candelli, PhD – Chief Scientific Officer and Founder
Rogier Reijmers, PhD – Principal Scientist
The Problem: Overcoming the Functional Limitations of NK Cell Therapies in Clinical Settings
Natural killer (NK) cell-based immunotherapies have shown promise in cancer treatment, but their clinical efficacy is often limited by poor persistence and reduced functionality, especially post-cryopreservation. These issues hinder their sustained activity, which is essential for long-term effectiveness. Memory-like NK (mlNK) cells have emerged as a potential solution, particularly in inducing complete remissions in hematological malignancies. Generated by exposing resting NK cells to IL-12/15/18 or co-culturing with specific tumor lines, mlNK cells retain potent cytotoxicity for up to 7 days, even after cryopreservation. However, the precise phenotypic and functional characteristics driving their enhanced efficacy remain unclear. Without a deeper understanding of the mechanisms behind this “memory” phenotype and a reliable method to generate these cells, translating NK cell therapies into effective treatments remains challenging. Addressing these issues is critical for unlocking NK cells’ full potential in cancer immunotherapy.
The Study: Identifying Crucial Biomarkers of Memory-Like NK Cells Using Cell Avidity
This study focused on uncovering the mechanisms that drive the enhanced cytotoxicity of mlNK cells, generated through both cytokine-induced (iCIML) and tumor-primed (TpNK) methods. By comprehensive analysis using high-dimensional flow cytometry, proteomic and metabolomic profiling, and cell avidity measurements—a critical component of this study—the researchers identified common characteristics that define NK cell memory and may underlie its improved efficacy in cancer cell killing.
Flow cytometry and proteomic analysis identified proteins uniquely associated with mlNK cells, many of which are involved in enhancing effector function and overcoming tumor-induced inhibition. Metabolomic profiling showed that these cells have increased glycolytic activity and improved mitochondrial fitness, enabling them to sustain their cytotoxic functions even in the challenging conditions of the tumor microenvironment.
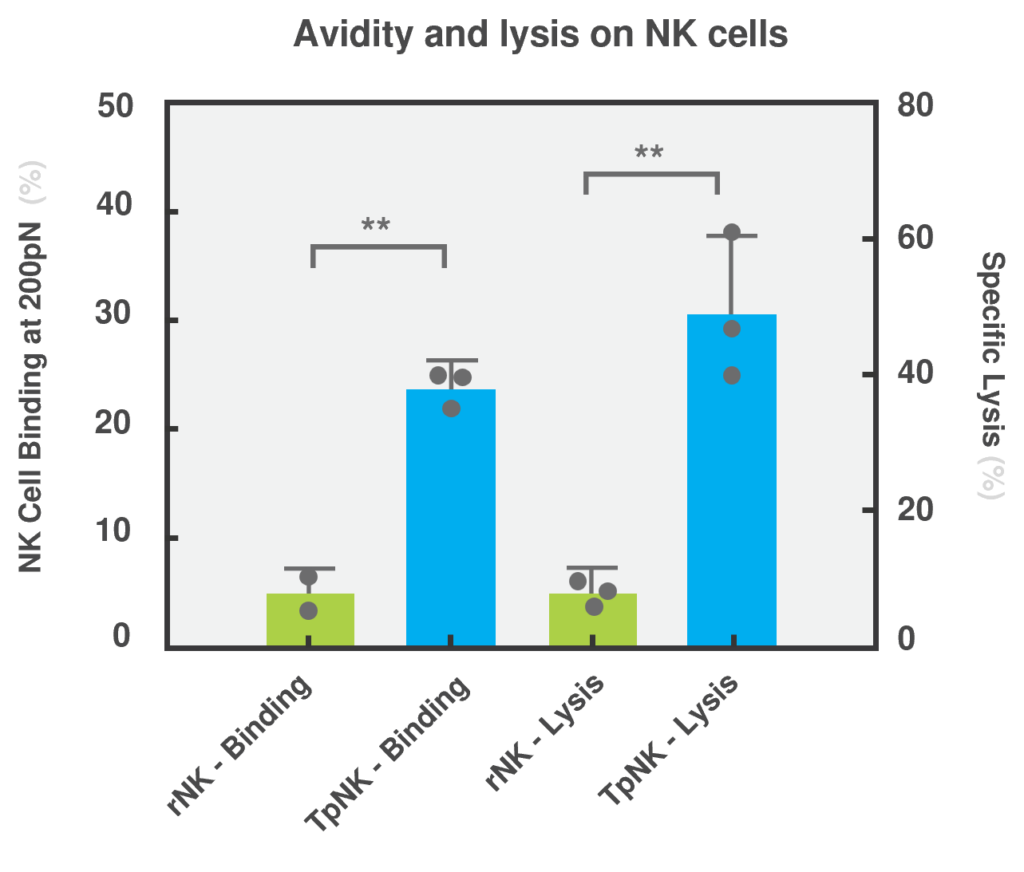
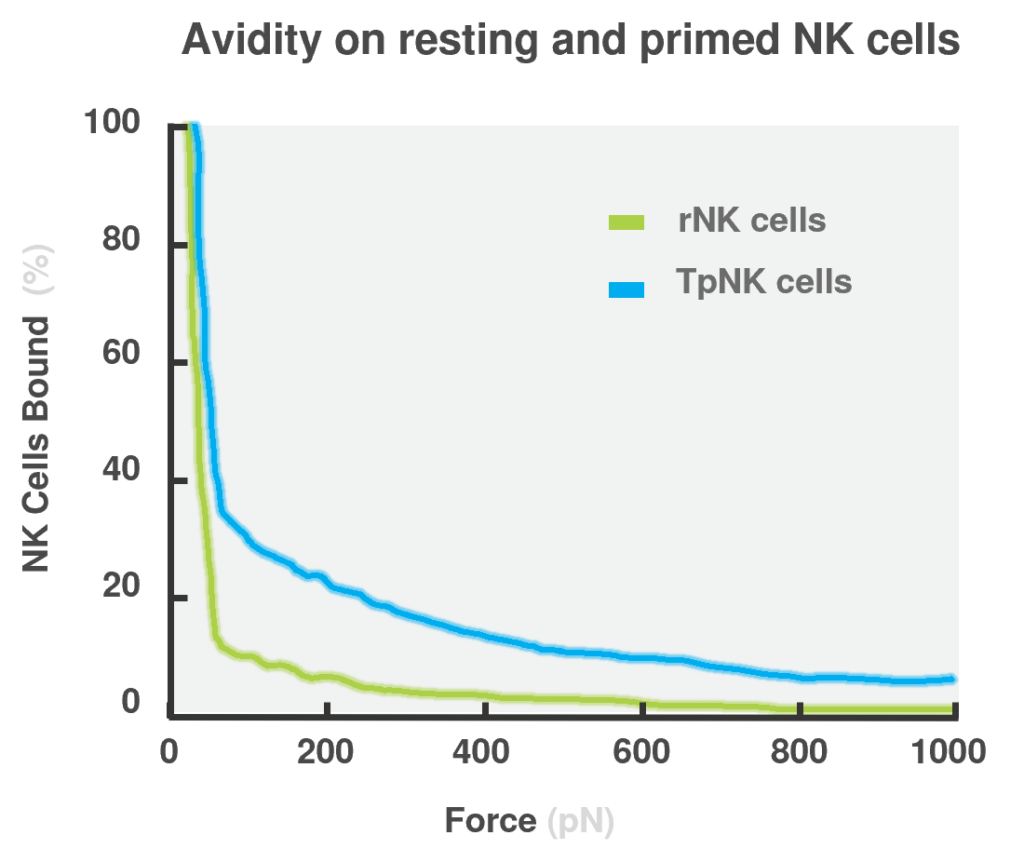
Data showing that tumor priming induces high avidity and high cytotoxicity in NK cells across different tumor types. (Arellano-Ballestaro, H. et al., 2024)
Crucially, cell avidity measurements using LUMICKS’ z-Movi technology demonstrated that binding to NK cell-resistant tumor targets was restored upon mlNK cell differentiation, while resting NK (rNK) cells were unable to bind to the same tumor types. This increased avidity strongly correlated with improved cytotoxicity in vitro, validating the hypothesis that strong binding enhances NK cell function, even to otherwise NK cell-resistant tumor cells. Importantly, the formation of a stable and mature immunological synapse, observed in high avidity mlNK cells, is a critical step for the successful delivery of lytic granules into the target cell.

Professor Mark W Lowdell
PhD, FRCPath, FRSB
Professor of Cell & Tissue Therapy
University College London
CSO and Founder at Inmune Bio
“Cell avidity was a central focus in our study, revealing crucial insights into how memory-like NK cells recognize, engage, and destroy tumor cells. Understanding the mechanism of action triggered by our NK cell pre-treatment gave us confidence in their enhanced targeted cytotoxicity. Our study identified cell avidity as a key predictor of in vitro function, offering a pathway for rapidly developing more effective NK cell-based immunotherapies.”
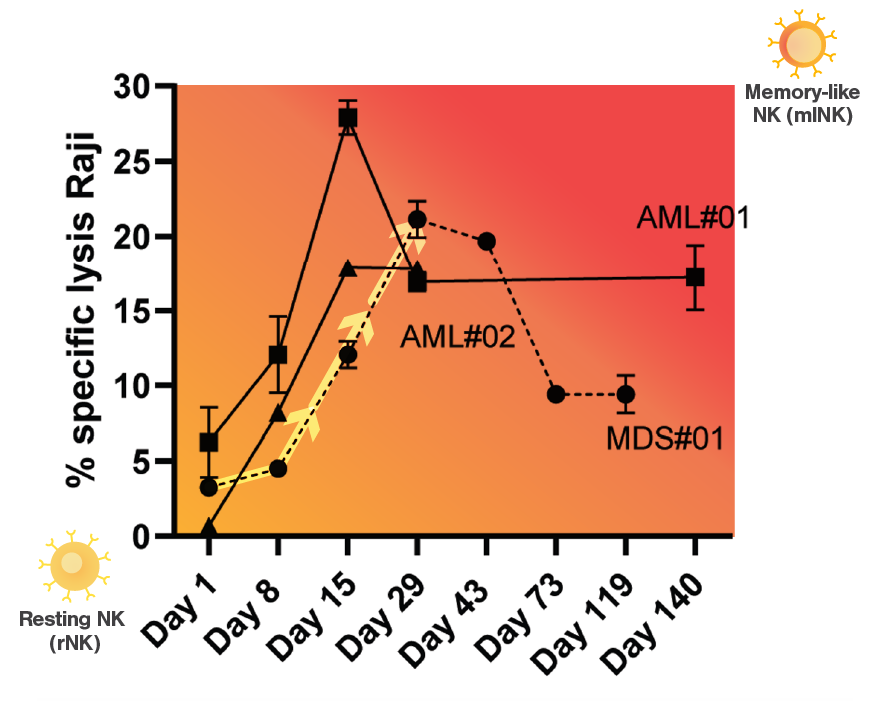
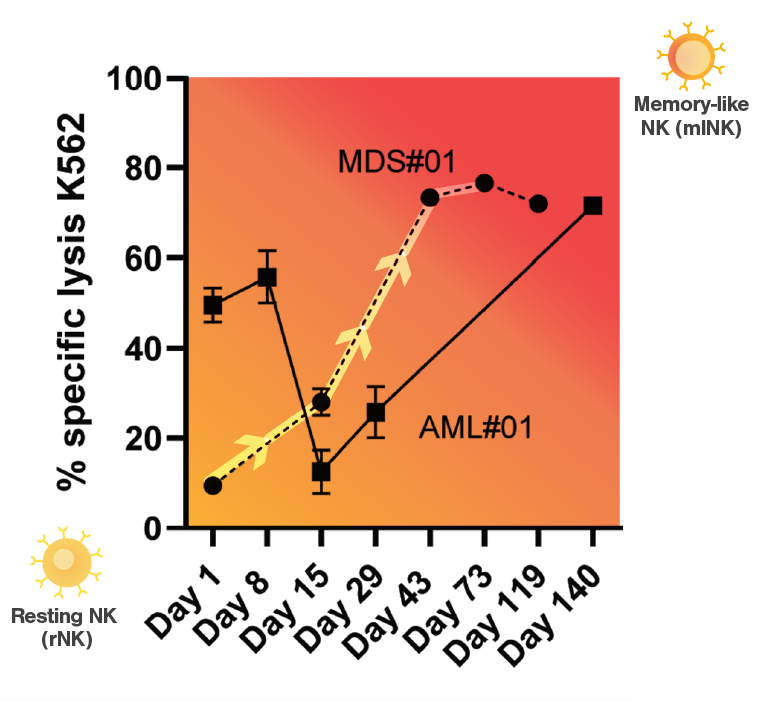
Specific lysis of the injected tumor cell lines Raji and K562 in three patients (AML#01, AML#02 and MDS#01) increases after treatment with INKmune, administered on days 1, 8, 15, 29 et cetera. This data confirms NK cell activation from resting NK cell to memory-like NK cell in patients. (Arellano-Ballestaro, H. et al., 2024)
The Implications: Enhancing NK Cell-Based Cancer Therapies by Avidity Characterization
This study’s findings have significant implications for cancer immunotherapy, particularly NK cell-based therapies. By introducing cell avidity as a biomarker, it offers a critical tool for predicting and improving mlNK cell therapies. High avidity not only indicates stronger NK cell-tumor interactions but also reflects the molecular mechanisms behind enhanced cytotoxicity against resistant tumors.
The study’s proteomic and metabolomic profiles reveal therapeutic targets that can boost NK cell-tumor interactions, enhancing metabolic fitness and resistance to tumor-induced suppression. Additionally, it highlights the need to refine manufacturing processes to consistently produce fitter, more effective NK cells.
Future research should validate these biomarkers and mechanisms in clinical trials to improve NK cell-based therapies and patient outcomes. The successful generation and maintenance of mlNK cells in patients, included in this study, opens new clinical applications, especially for treating hematological malignancies and resistant cancers.
The Broader Context: Harnessing the Full Potential of NK Cells in Cancer Immunotherapy
Natural Killer (NK) cells are a vital component of the innate immune system, known for their ability to recognize and destroy abnormal cells, including cancer cells. Unlike T cells, which require specific antigen recognition to mount an immune response, NK cells can identify and eliminate tumor cells based on the absence of ‘self’ markers, such as MHC class I molecules, and by recognizing metabolic and other biological signatures of stressed or abnormal cells. This dual recognition makes NK cells a powerful tool in targeting cancer, particularly in scenarios where tumors evade T-cell detection.
NK-cell-based cancer therapy leverages these unique properties to target a wide range of malignancies, offering a promising approach to treating both hematological and solid tumors. Over the past few years, advances in NK cell therapies, including the development of memory-like NK (mlNK) cells, have shown significant potential in overcoming some of the limitations faced by traditional cancer treatments, such as chemotherapy and T-cell-based immunotherapies.
Broader Targeting Potential
NK cells can target a wide range of tumor cells, thanks to their natural cytotoxicity and ability to detect stressed or abnormal cells. Unlike CAR T-cells, which must be engineered to recognize specific antigens, NK cells can identify and destroy a variety of cancer cells without the need for prior sensitization. This broad targeting capability makes NK cells a versatile and effective option for treating various types of cancer, including those where specific antigens are not well-defined or are prone to mutation.
Lower Risk of Severe Side Effects
One of the most significant advantages of NK cell therapy is the favorable safety profile, particularly when compared to CAR T-cell therapy. NK cells are less likely to cause severe immune reactions, such as cytokine release syndrome, which is a common complication of T-cell-based treatments. This makes NK cell therapies safer and more manageable in a clinical setting, offering a potentially life-saving option for patients who may not tolerate the intense side effects associated with other forms of immunotherapy.
Suitability for Off-the-Shelf Therapies and Reduced Risk of Graft-Versus-Host Disease (GVHD)
NK cells are uniquely suited for off-the-shelf therapies due to their lower risk of graft-versus-host disease (GVHD) because of their transient presence compared to T cells. This advantage stems from their role in the innate immune system, which does not require specific antigen recognition to function. As a result, NK cells can be harvested from donors and used to treat multiple patients without the need for extensive matching, making them a scalable and practical option for widespread clinical use. Their ability to function without specific antigen recognition reduces the likelihood of harmful immune responses, making NK cells safer for use in allogeneic transplants.
Notable Researchers: Renowned scientists utilizing Cell Avidity for developing NK-based Therapies
1. Prof. Mark Lowdell, Cancer Institute, University College London, London, UK
Prof. Mark Lowdell is a leading figure in the field of immunotherapy, serving as the Director of Cellular Therapeutics at University College London (UCL). With over two decades of experience, Lowdell has been at the forefront of research in NK cell-based therapies, focusing on the development and clinical application of novel cellular immunotherapies. His pioneering work integrates cell avidity measurements to enhance the efficacy of NK cells, particularly in the treatment of hematological malignancies, making significant strides in translating benchside research into bedside treatments.

Access Prof. Mark Lowdell's exclusive webinar
2. Dr. Peter Chockley, St. Jude Children’s Research Hospital, US
Dr. Peter Chockley, a talented researcher at St. Jude Children’s Research Hospital in the lab of Stephen Gottschalk, M.D., is at the forefront of CAR-NK cell immunotherapy research. His work focuses on enhancing the efficacy of CAR-NK cells in targeting and eliminating cancer cells. A pivotal aspect of his research involves measuring cell avidity: “Most influential were the avidity measurements that directly interrogated the strength of synapse.” This approach has proven to be a critical predictive parameter for in vivo tumor control, offering promising advancements in the fight against cancer.