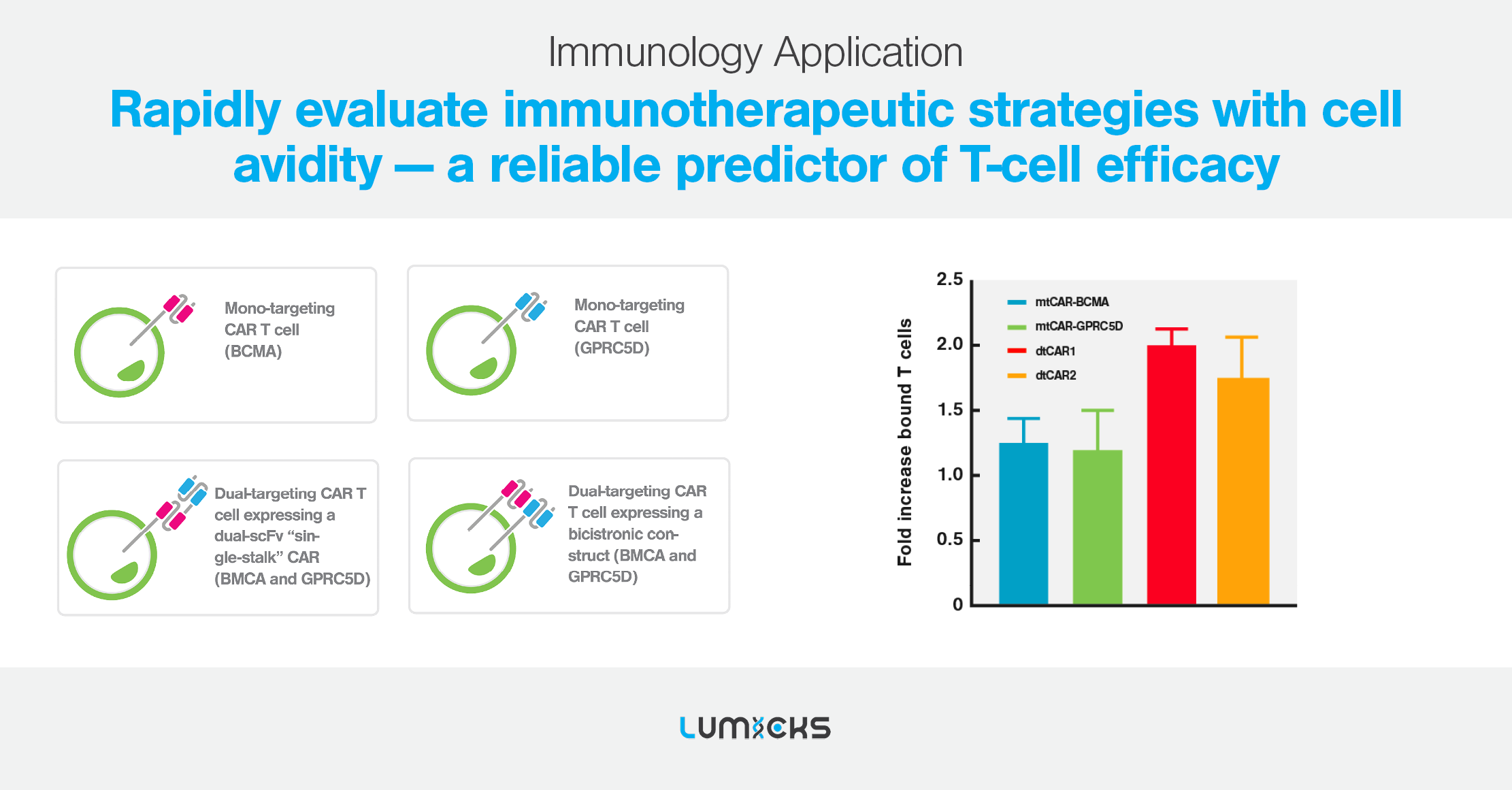
NEW: Cell Avidity identified the poor clinical efficacy of the APRIL CAR-T cell therapy
In a recent JITC paper named “Limited efficacy of APRIL CAR in patients with multiple myeloma indicate challenges in the use of natural ligands for CAR T cell therapy’’ published by the team of Dr. Lydia Lee, cell avidity successfully identified the issue behind the poor clinical efficacy of the APRIL CAR shown during an unsuccessful phase I clinical trial to treat multiple myeloma while traditional in vitro assays failed to pinpoint the issue (Figure 1).
Cell avidity enabled researchers to establish poor tumor binding (or avidity) by cell bound APRIL compared to competitor FDA approved CARs (Figure 2) as a likely explanation providing an improved preclinical readout that can predict efficacy in patients.
Key takeaways:
- APRIL CAR had significantly reduced binding to BCMA-expressing target cells, ranking the lowest among the FDA-approved clinical CARs (bb2121 and LCAR).
- This deficiency in binding contributed to the APRIL CAR’s poor performance in the phase I clinical trial.
- Incorporating cell avidity would have allowed for more informed decision-making, potentially leading to the selection of a different lead candidate or optimizing the APRIL CAR design before its clinical trial deployment
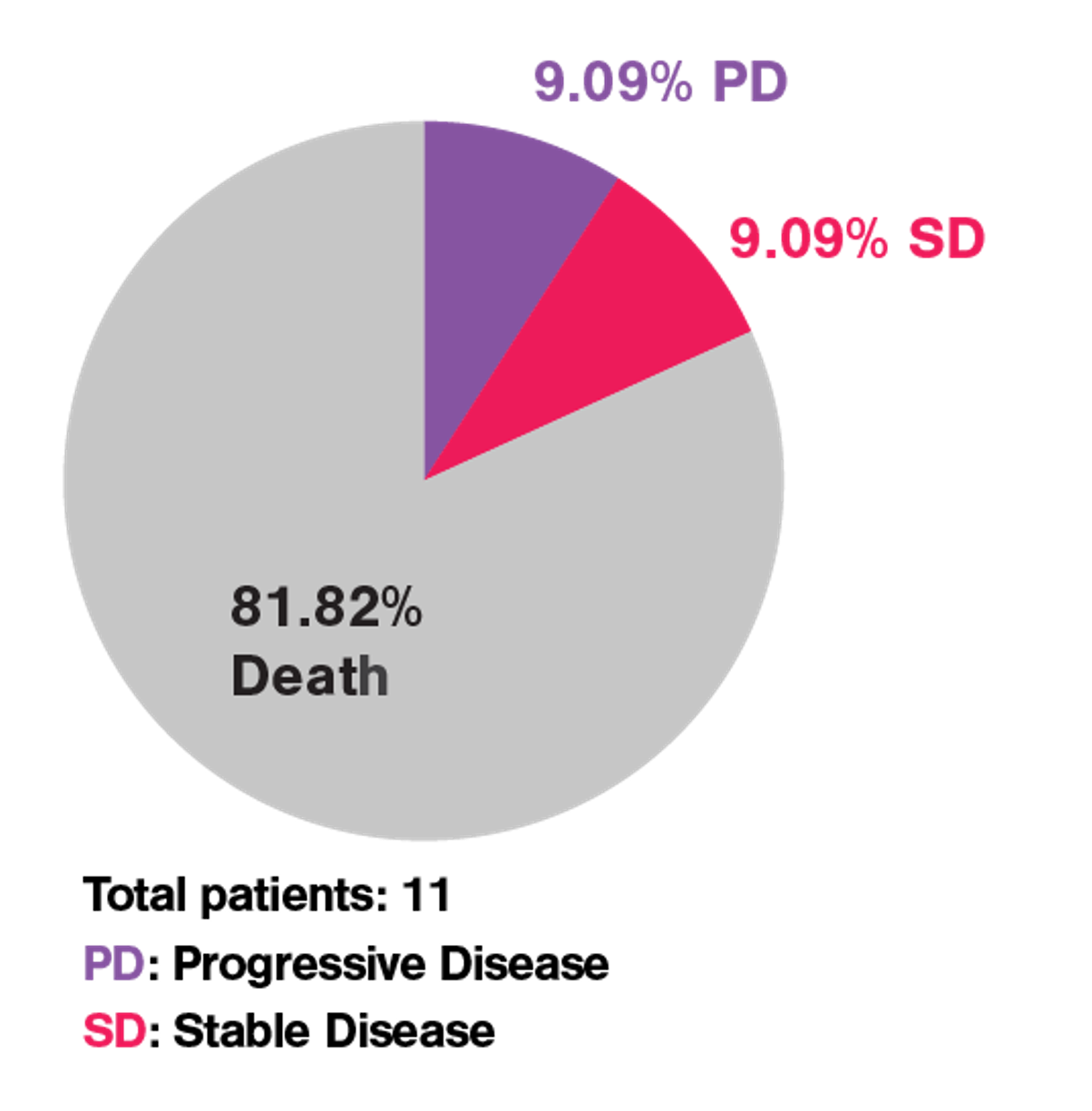
Figure 1 Phase I clinical trial of APRIL CAR-T cells in patients with refractory MM resulted in poor clinical outcome.
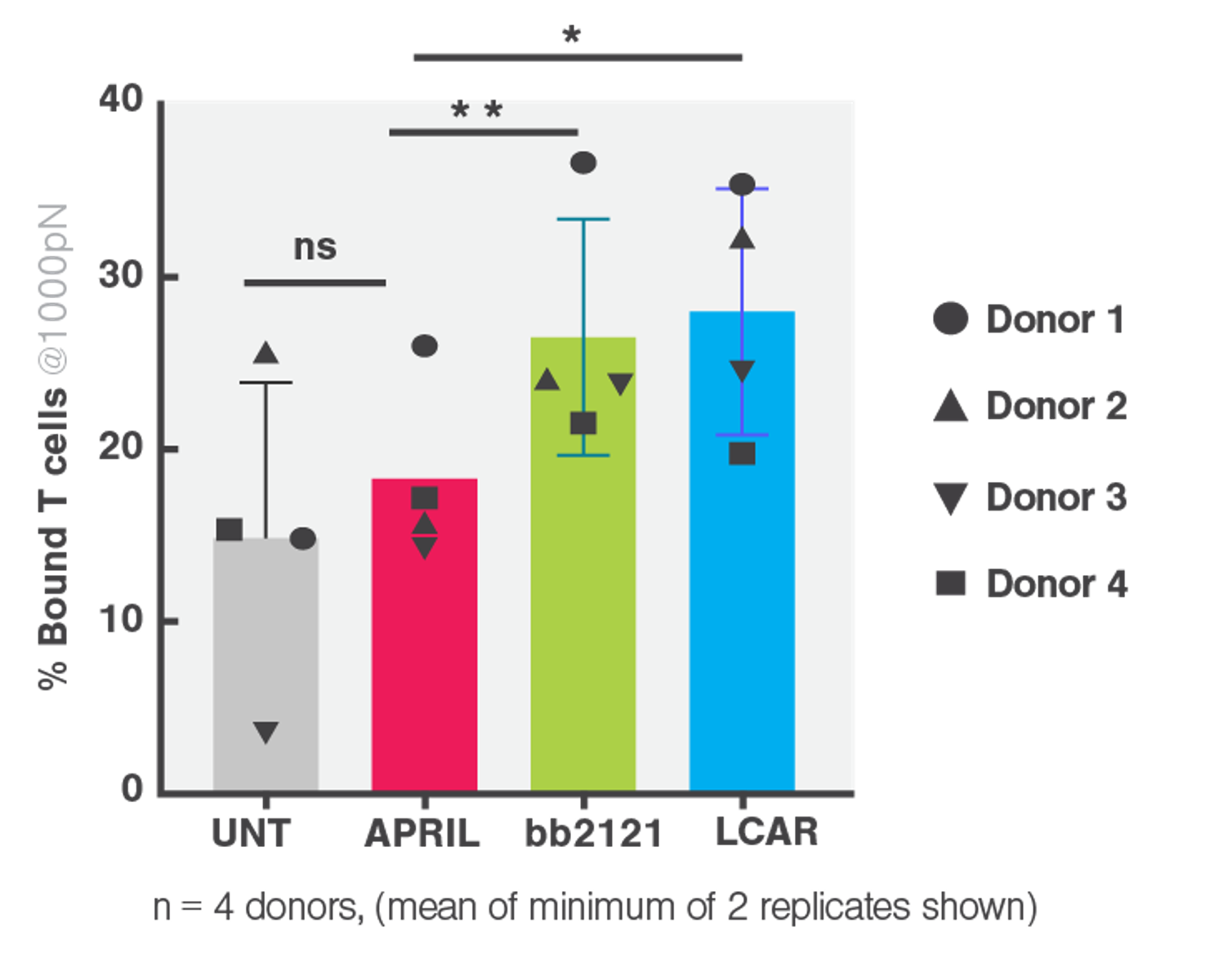
Figure 2 Cell avidity between CAR transduced T cells from 4 healthy donors and myeloma cell line H929 gated at 1000 pN. Multiple paired T tests and Holm Sidak correction *p<0.05, **p<0.01, ***p<0.001.

Dr. Lydia Lee
University College London
Group Leader Cell Therapy
‘’In this unique exploration of the reasons underpinning suboptimal clinical responses in the AUTO2 trial, the LUMICKS platform allowed us to establish poor tumour binding (or avidity) by cell bound APRIL compared to competitor CARs as a likely explanation. Only with such studies will the field acquire a greater understanding of the key preclinical readouts that can predict efficacy in patients.’’
Traditional screening methods give an inaccurate indication of CAR T efficacy in vivo
Recent research findings from Dr. Marcela Maus’ group uproot the current ways used to select CAR variants for cancer therapies while presenting a new, highly predictive biomarker. This research shows how current in vitro screening methods do not accurately predict in vivo efficacy, where as cell avidity does.
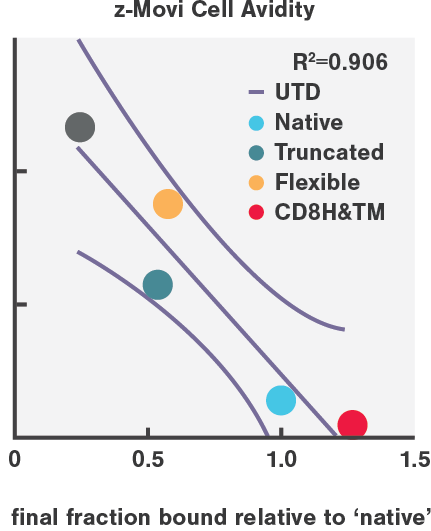
CAR-T cell avidity variants highly correlated with in vivo tumor eradication efficiency (R=0.906), in high contrast to in vitro cytotoxicity (R=0.598) and cytokine secretion.
Enhanced tumor clearance in AML mouse models results from enhancing the binding avidity on both sides of the synapse, by engineering more stable CD27-based CARs and pharmacologically increasing CD70 antigen density on AML cells.
Watch our Nature webinar!
Cell Avidity Measurements to Understand and Enhance CAR-T/Tumor Interactions in Acute Myeloid Leukemia
Dr. Marcela Maus used avidity measurements to understand and enhance productive CAR-T cell interactions.
Solid tumors avoid CAR T cell killing through cell avidity escape
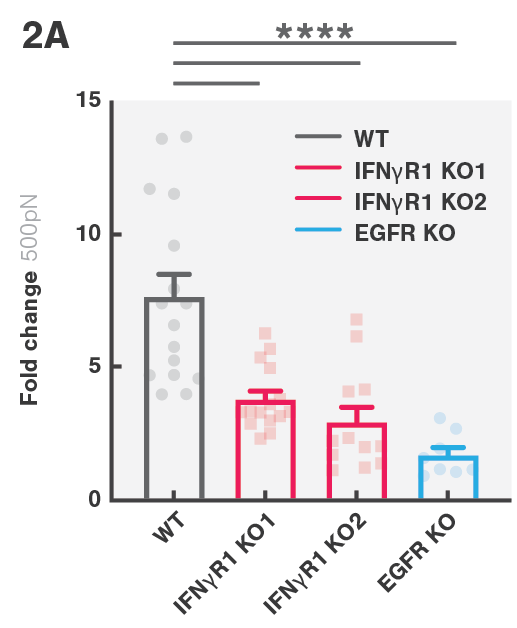
CAR T cell therapies have been transformative in battling blood-related cancers; however, the more prevalent solid tumors have been less responsive to this therapeutic approach. This study aimed to investigate the mechanisms involved in solid tumor escape of CAR T cell therapies. By screening several glioblastoma cell lines, including ones derived from patients, the team of Dr. Marcela V. Maus found that loss of genes in the interferon-γ receptor (IFNγR) signaling pathway (IFNGR1, JAK1 or JAK2) rendered them more resistant to CAR T cell killing. Similar results were observed in vivo in IFNγR knockout mouse models.
Transcriptional profiling of IFNγR knockout (KO) cells showed a lower upregulation of cell-adhesion molecules after exposure to CAR T cells, leading to reduced cell avidity (Figure 2A) and reduced CAR T cell killing. This, ultimately, resulted in solid tumor escape. The avidity escape resistance mechanism to CAR T cell killing was further established by showing that ICAM-1 overexpression in IFNγR1 KO cells restored cell avidity.
Watch our Nature webinar!
Cell avidity as a crucial biomarker for CAR T response
Dr. Rebecca Larson’s work revealed important evasion mechanism from CAR T cell response in solid tumors.
Our solutions
Cell avidity as a service
Cell Avidity analysis can be performed at high throughput using our services platform, enabling the screening of hundreds of CAR-T cells to rapidly find the most potent CAR-T for in vivo testing.
Dr. Rogier Reijmers
Principal Scientist at LUMICKS