
Molecular biology as never seen before
The world’s first dynamic single-molecule microscope to allow simultaneous manipulation and visualization of single-molecule interactions in real time.
Highly detailed results at high throughput
Manipulate your sample with exceptional stability and precision, utilizing powerful automation features and extensions.
Easy to learn workflow & software
Start experimenting right away using our conventional microfluidics and software solutions designed specifically for C-Trap experiments.
Purpose-fit for your needs
Choose from a wide degree of optical trap and imaging wavelength configurations to fit your workflow.
A typical experiment
High-throughput experiment workflow

Load your samples
Load your samples and conditions into the syringes of the 5-channel automated microfluidics system. Pipetting each reagent takes seconds thanks to the twist-and-lock syringe adaptor, with which you can quickly and easily refill individual syringes.

Assemble your assay without physical barriers
Seamlessly move the optical traps within the laminar flowcell to catch beads, move them between the 5 microfluidics lanes, and assemble your constructs.

Perform and automate your experiments
Gather statistically relevant quantities of data and publishable results with ease. The C-Trap allows for automation with full access to all relevant system parameters and data streams.
With an experimental run taking less than 80 seconds, including automated assay assembly, it's possible to get 18 useable sets of data within half an hour.

Organize and analyze your data
View, compare, and export your fully correlated data streams. The C-Trap stores all your metadata so you never lose valuable information, and always have the option to reproduce your experiments.
Our analysis software comes with tutorials and sample notebooks that can serve as a scaffold for your own analyses. In addition, we have an open-source platform where you can upload, download, and review user scripts for free.
Take a look inside
Discover the manipulation and imaging technologies that bring the C-Trap to life
Precise sample manipulation
Study the smallest of interactions using optical traps combined with advanced software features. Available in multiple configurations, the C-Trap can handle a broad variety of assays.
Multicolor fluorescence imaging
Visualize biological processes such as protein kinetics and (un)binding events on DNA or measure conformational changes of proteins by combining the C-Trap with FRET. Our fast 1D scanning capabilities make it excellent for constructs such as DNA or filaments.
Laminar microfluidics
Assemble your assay and study a variety of proteins or conditions in the same experiment with our our dedicated laminar flow cell and automated microfluidics solution.

Data acquisition and analysis tools for every type of (single) molecule
Execute and analyze your C-Trap experiments using our intuitive Bluelake & Lakeview software.
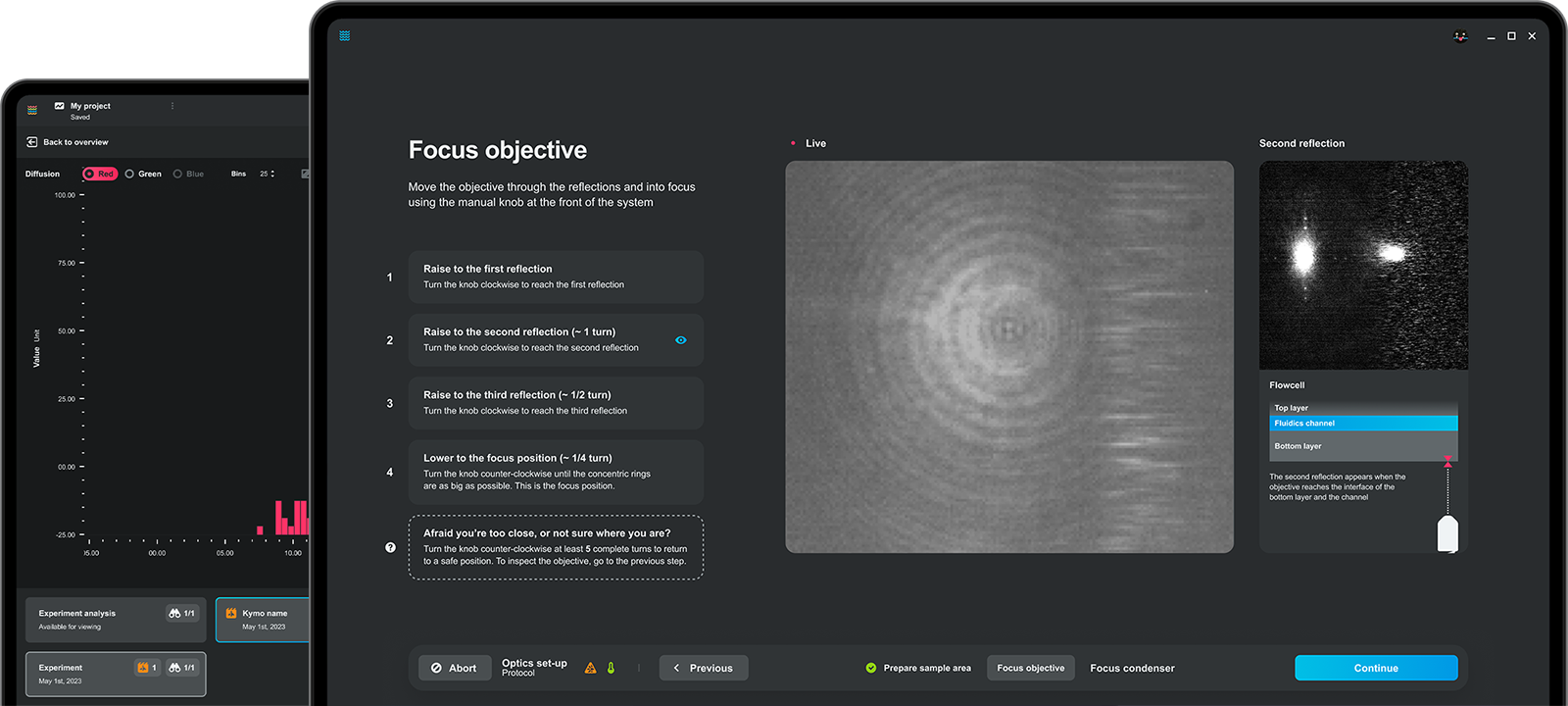
Execute and automate your experiment with ease
Adapt and iterate on the fly with real-time data
Obtain immediate insights and study them wherever, whenever
Download the brochure
Get the latest all-in-one overview

C-Trap Accelerator Suite
Supercharge your C-Trap into a faster, easier, more reproducible powerhouse. C-Trap Accelerator Suite boosts the capabilities of the instrument you already own, letting you capture publishable single-molecule data in half the time with rock-solid reproducibility.

Beyond the product
Take a look at the perks that come with being one of our users

Join the community
SMBIO (Single-molecule Biology) is LUMICKS’ symposia format. We regularly host events across the world, aiming to bring together scientists interested in dynamic single-molecule applications to share their research, connect, and exchange ideas and experiences.

Ready-made reagent kits and tailored sample preparation services
Use our biochemistry platform and internal expertise for the purification, labeling, and preparation of your reagents for optical trapping experiments. With our multidisciplinary expertise in molecular biology, biochemistry, and dynamic single-molecule analysis, we’ve created the most extensive selection of ready-made reagents, kits, and tailored services to prepare your samples.

Your success is our success
Our goal is to minimize your time to results, ensure success, and provide instruments that are easy to use and maintain. To this end we have dedicated teams providing user training, application support and system check-ups.
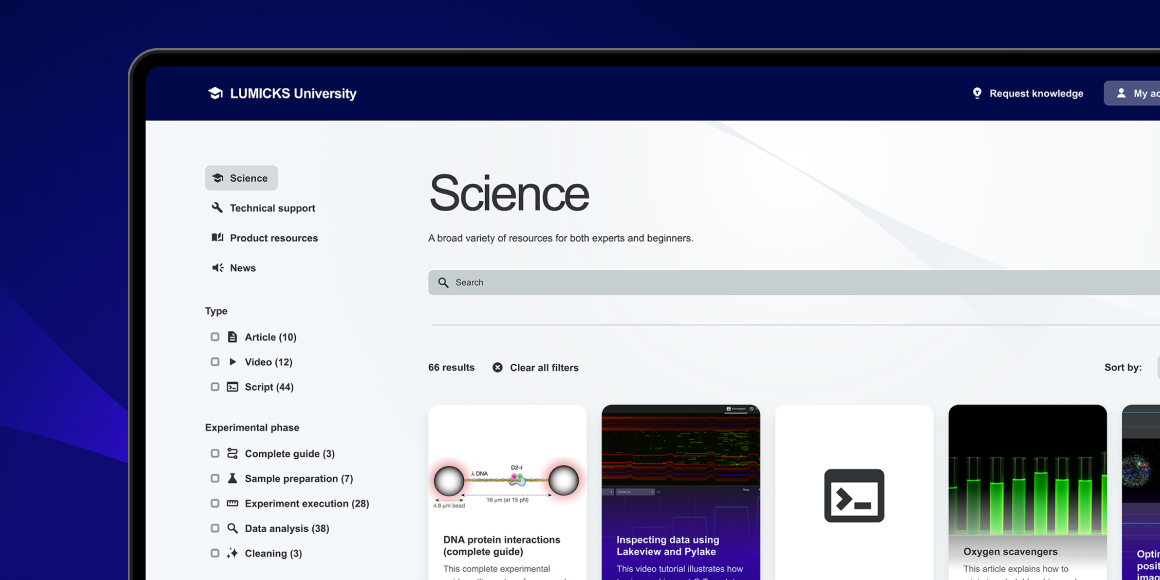
Your gateway to mastering single-molecule experiments
LUMICKS University is our all-in-one learning hub to simplify your learning, deepen your knowledge and accelerate your discoveries, designed exclusively for LUMICKS customers.
Let’s begin your LUMICKS journey
Our experts are ready to learn about your research challenges and see where our technologies can bring value.
Demonstrating value
Our application scientists can help create interest among potential users through organizing different events such as seminars, workshops, and demonstrations, as well as meet with stakeholders individually to understand and help solve their needs.
Grant & tender support
Throughout our history we have supported multiple successful grants across a broad spectrum of funding and users involved. Our application scientists are experienced in highlighting the unique value of Dynamic Single-molecule and its solutions, and are able to collect proof-of-concept data to strengthen your grant application.

