Keeping up with the complexity of binding mechanisms
- Generate direct, physiologically relevant measurements of binding in its full, dynamic complexity
- Understand the mechanism of action of your therapeutic products by revealing its complex binding dynamics
- Balance potency and safety by optimizing binding
Enhancing efficacy against clear cell renal cell carcinoma through format-tuning of bispecific T cell engagers
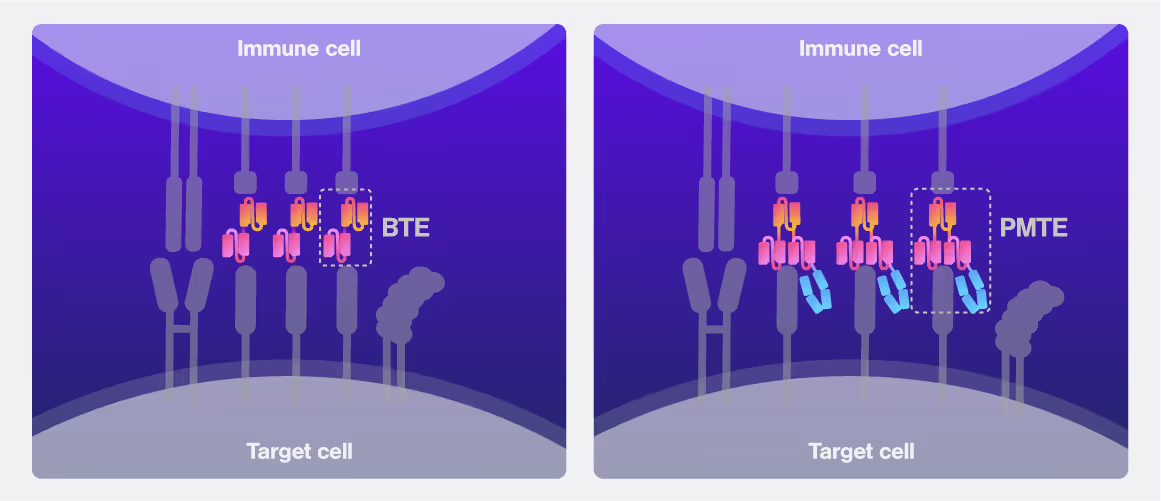
First-generation bispecific T cell engagers (BTEs) have shown promise in preclinical models but often suffer from a short plasma half-life due to their small size and absence of an Fc domain. Additionally, limited tumor retention can lower local therapeutic concentrations and impede efficacy. Cancer cells can even adapt and evade immune targeting through antigen heterogeneity and loss, threatening sustained responses. The immunosuppressive tumor microenvironment further hinders T cell function and promotes therapeutic resistance. To develop an effective strategy, any cell engager must overcome these barriers to achieve robust, consistent targeting and immune activation.
A team led by David Weiner, PhD have examined how format-tuning bispecific T cell engagers (BTEs) boosts therapeutic efficacy against clear cell renal cell carcinoma (ccRCC). Using a novel persistent multivalent T cell engager (PMTE) to enhance cell avidity and tumor targeting, they tackle challenges like low plasma half-life, poor tumor retention, and antigen escape. Optimizing cell interactions through avidity-driven design offers a pathway to more effective, durable cancer therapies and renewed hope for advanced ccRCC patients.
Cell avidity curves represent the % of target cells bound with rising detachment force measured at 30 nM, 3 nM and 300 pM antibody concentration. Area-under-curve quantifications indicate the significant differences in cell avidity between PBTE and PMTE at relevant concentrations. Adapted from: O’Connell et al. (CC-BY-NC)
Dive into the publication
Format-tuning of in vivo-launched bispecific T cell engager enhances efficacy against renal cell carcinoma
Avidion
The next generation Cell Avidity platform


Avidigo
White glove Cell Avidity services

z-Movi
For small sized Cell Avidity studies
These cards are NOT components because they use the finsweet nested collection logic. To pull in the posible multiple people wo worked on it.
This type of 1-many relation is not supported native in Webflow.
Also this section is hidden when emtpy. To keep everything visible here, that is being done outside the webflow designer from within Slater.
SITC 2025
CAR-TCR Summit 2025
CICON 2025



.avif)
.avif)



