Unveiling the Hidden Exchange Dynamics of DNA Polymerases
For decades, researchers have been on a quest to unravel the elusive mechanisms governing DNA polymerase exchange during replication—a process essential for genomic integrity and genome replication. Despite the central role these enzymes play in life itself, understanding how they manage rapid dissociation and reassociation at the replication fork without compromising fidelity has remained one of molecular biology’s greatest challenges.
Basic Mechanisms and Key Proteins in DNA Replication
DNA polymerases are crucial enzymes that catalyze synthesis of new DNA using on single-stranded DNA (ssDNA) as template. These enzymes are central to the duplication of genetic material, playing a pivotal role in the propagation and maintenance of life.
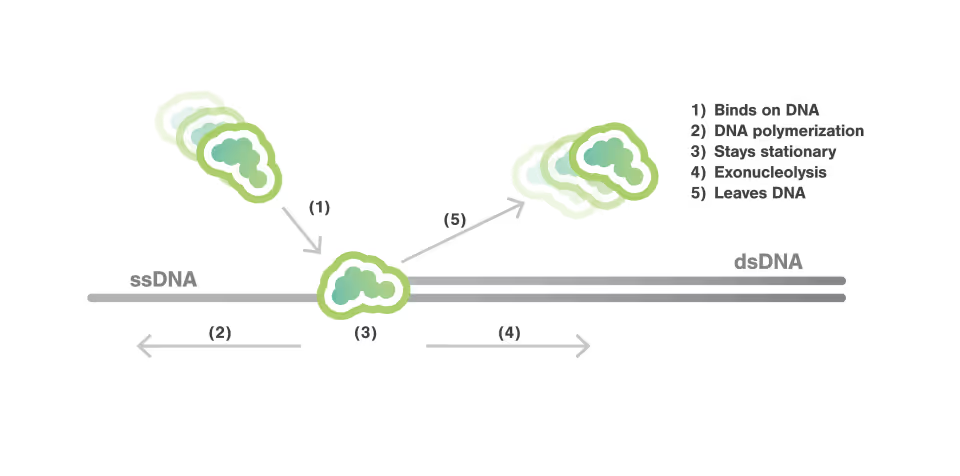
During DNA replication, DNA polymerases do not continuously remain bound from the initiation to the termination of replication process. Instead, they rapidly associate with and dissociate from the DNA, a process known as DNA polymerase exchange.
However, the mechanisms and kinetics underlying polymerase exchange on DNA are not well understood nor characterized, particularly regarding the kinetics of the polymerase exchange and how it affects the activity and overall fidelity and dynamics of the replisome.
Key Proteins Involved in DNA Replication
DNA Polymerase – Enzymes responsible for synthesizing new DNA strands.
Helicase – Unwinds double-stranded DNA into single strands, allowing access for the replication machinery.
Primase – Synthesizes a short RNA primer on the DNA template, providing a starting point for DNA polymerases.
Sliding Clamp – Encircles DNA and holds the DNA polymerase in place during replication, increasing its processivity (PCNA – Proliferating Cell Nuclear Antigen)
Clamp Loader – This complex loads the PCNA onto the DNA (RFC – Replication Factor C)
Topoisomerases – These enzymes relieve the tension generated by the unwinding of DNA, preventing supercoiling and possible breakage.
Single-Stranded DNA-Binding Proteins (SSBs) – Bind and stabilize the exposed single-stranded DNA until it can be used as a template for replication.
DNA Ligase – Seal the nicks between the Okazaki fragments on the lagging strand, creating a continuous double-stranded DNA molecule.
Replication Protein A (RPA) – Stabilizes single-stranded DNA and is essential for replication to proceed.
MCM Complex (Minichromosome Maintenance Complex) – Functions as the helicase in eukaryotes, it is crucial for the initiation and elongation phases of DNA replication.
The study: using Dynamic Single-Molecule Methods to unravel DNA polymerase dynamics
A recent study from Gijs Wuite’s lab at VU University Amsterdam has made significant advancement in understanding DNA polymerase dynamics during replication.
The researchers employed the C-Trap technology to monitor DNA polymerase dynamics. The enzymatic activity of DNA polymerase was detected by observing changes in DNA length, which occur due to the differential elasticity of single-stranded DNA (ssDNA) and double-stranded DNA (dsDNA). While this change in length indicated polymerization or exonucleolysis activity, the engagement of the polymerase at the junction was simultaneously detected using fluorescence. This dual approach provided a comprehensive view of how DNA polymerases bind, dissociate, and perform their enzymatic functions during DNA replication.

Limitations of Traditional Tools in Studying DNA Polymerase Dynamics
Cryo-Electron Microscopy (Cryo-EM):
Cryo-EM excels in providing high-resolution structural snapshots of biomolecules. However, it captures only static images, freezing DNA polymerases in a singular moment without revealing the dynamic binding processes that occur during replication. This lack of temporal resolution leaves the rapid exchange dynamics of polymerases at the replication fork largely unexplored.
Live-Cell Imaging:
Live-cell imaging offers the advantage of observing cellular processes in their natural environment, allowing for the visualization of DNA replication in real-time. However, its limited spatial and temporal resolution means it cannot effectively track the rapid binding and dissociation events of individual DNA polymerases at the replication fork. As a result, critical details about the polymerase exchange process remain obscured.
Bulk Biochemistry Assays:
Bulk biochemical methods have been fundamental in understanding DNA replication, offering insights into enzyme kinetics and interactions. Yet, these methods average the behaviour of millions of molecules, masking the individual dynamics of DNA polymerases. This averaging effect makes it impossible to distinguish the specific events of polymerase exchange and their role in maintaining replication fidelity.
Key Findings and scientific insights:
The study characterized the kinetics of polymerase exchange, revealing the exact timing employed by DNA polymerases to rapidly bind and dissociate from the DNA during replication. They directly observed that the bound-lifetime duration of a DNA polymerase to be in the order of 1 second, demonstrating that DNA polymerases only transiently associate with DNA.
For the majority of its bound-lifetime time, the DNA polymerase was actively engaged in either synthesizing new DNA or removing nucleotides. Besides, for over 90% of the time, the polymerase maintained a single type of activity during each binding event.
Switch in the mode of activity a binding event, such as transitioning from an active state to a paused state, or vice versa, were not observed frequently. This suggests a high level of task specificity and efficiency in the T7 polymerase’s interactions with DNA.
Additionally, during periods of polymerase or exonuclease activity, the authors observed multiple bursts of fluorescence. This indicates that the T7 polymerase repeatedly binds to and dissociates from the DNA within a single continuous activity cycle. Such observations provide direct evidence of active polymerase rapidly exchange during enzymatic processes, highlighting the dynamic nature of DNA replication and repair mechanisms.
Further analyses demonstrated that T7 DNA polymerase exhibits a “memory” of its previous activity states during exchange. If the polymerase was actively synthesizing or excising nucleotides before dissociating from the DNA, it typically resumed these activities upon re-association. Similarly, if a polymerase entered a pause state during a prior engagement, it tended to remain paused when it reattached in subsequent interactions.
This persistence of activity state suggests that the transition between active and inactive phases is not random but is influenced by the polymerase’s last known state, indicating a likely influence of DNA topology on this “memory” effect.
The Broader Implications: Understanding Life and Disease
These observations offer profound insights into the molecular mechanism of DNA polymerase exchange activity. Notably, the enzyme can autonomously bind and unbind from DNA without assistance from other proteins. Each binding event typically involves the polymerase performing a singular, specific task. Moreover, when a polymerase binds or unbinds, the system tends to preserve the previous activity status. These findings enhance our understanding of DNA replication.
The unique kinetics-function assay facilitated by this methodology is not achievable through any other existing technology, underscoring its significance in advancing our comprehension of molecular biology.
Images are adapted from the original publication: Xu L, Halma MT, Wuite GJ. Mapping fast DNA polymerase exchange during replication. Nature Communications. 2024 Jun 22;15(1):5328. https://doi.org/10.1038/s41467-024-49612-3