Did you know that cell avidity, a measure of binding probability between T cells and tumor cells, can be used to identify CAR-T products that are both potent and safe?
Next generation chimeric antigen receptor (CAR) T cell therapies holds the promise in providing a curative solution for solid tumors. Nonetheless, the significant clinical hurdle of on-target off-tumor toxicity (OTOT) remains a major impediment to the advancement of CAR-T therapies. Unlike hematological malignancies where CAR-T therapy has achieved remarkable results due to the presence of tumor-specific targets, solid tumors generally express tumor-associated antigens (TAAs), that are also present at lower levels in healthy tissues. This can lead CAR-T cells to inadvertently attack these non-malignant cells, thus hampering the translation of CAR-T therapy’s success from blood cancers to solid tumors.
Among solid tumors, clear cell renal cell carcinoma (ccRCC) is particularly relevant due to its prevalence, aggressiveness, and high rate of metastasis. Resistant to conventional chemotherapy, ccRCC has pushed the scientific community to explore innovative CAR-T treatments. CAIX is one of such tumor-associated antigens (TAAs): is highly expressed in ccRCC but also found in non-malignant tissues such as the bile duct and small intestine. Previous trials targeting CAIX, faced significant setbacks including severe OTOT toxicity and inadequate T cell persistence. The adverse effects were primarily due to the CAR-T cells targeting low levels of CAIX expressed on healthy bile duct cells, which led to the discontinuation of treatment for half of the patients.
Such termination exemplifies not only the need for CAR-T therapies that can precisely target tumor cells, while sparing healthy ones, but also highlights significant gaps in the current toolkit for early-stage CAR T safety assessment. The complex interplay required in the design, screening, and selection of therapeutic CARs becomes especially critical for solid cancers like ccRCC, where balancing potency and safety is essential. Therefore there is a urgent need of new and innovative methods to streamline the assessment of potential toxicities early in the development process, ensuring safer and more effective treatments
Dana-Farber breakthrough enabled by avidity tuning for improved safety of CAR T therapy in solid tumors
Researchers from the Dana Farber Cancer Institute at Harvard Medical School have developed a cell avidity-based pre-clinical analysis pipeline for CAR therapeutic selection (Wang et al., Molecular Cancer, 2024). This innovative pipeline integrates both safety and potency assessments, utilizing the innovative LUMICKS’ z-Movi Cell Avidity Analyzer to evaluate a panel of CAR candidates for ccRCC cancer. The Harvard-based team demonstrated a key advancement with their newly developed G9 anti-CAIX CAR product, which offers potential enhancements in treatment options for kidney cancer patients.
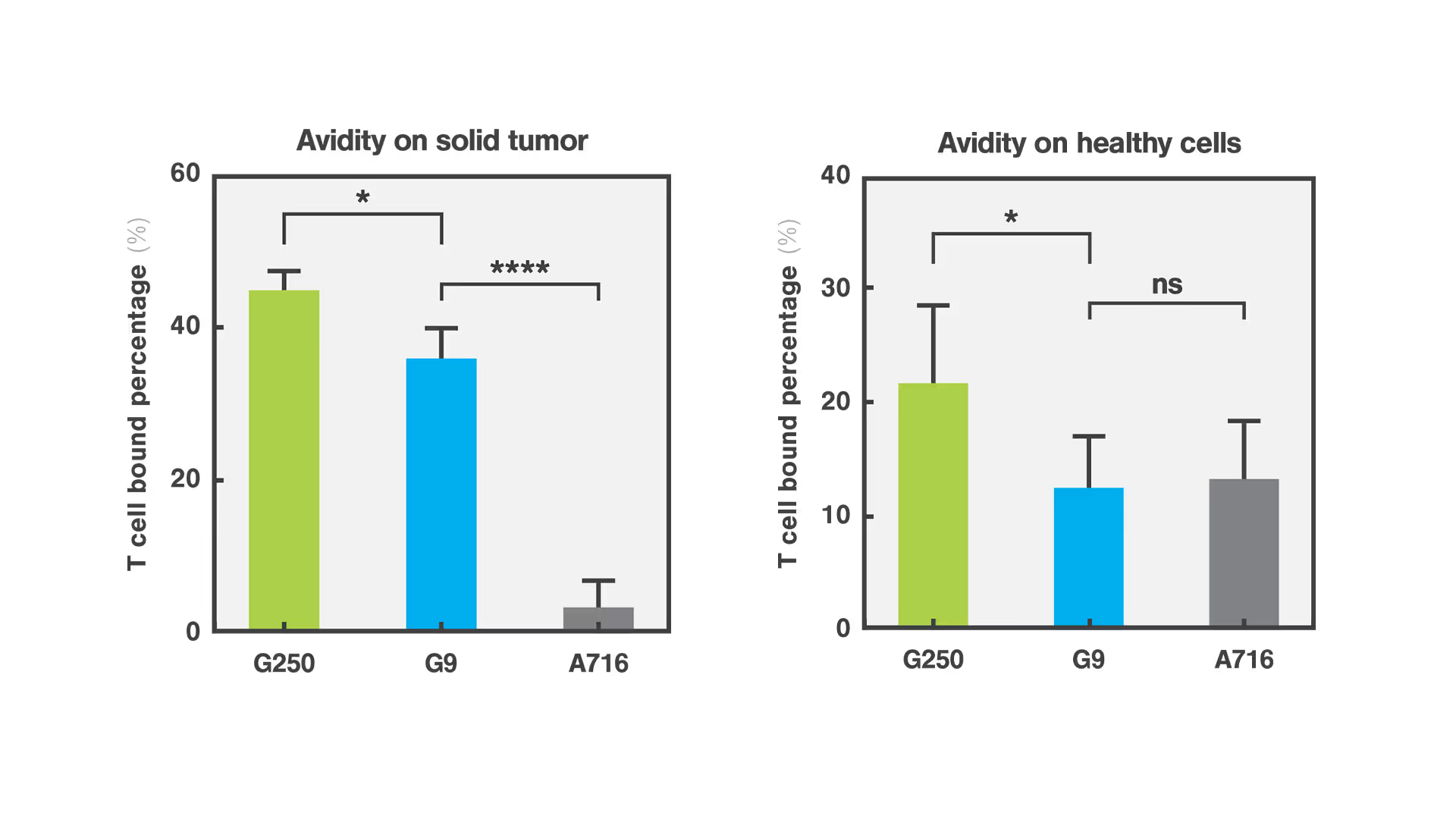
The G9 CAR, despite its low binding affinity for the CAIX molecule, demonstrated strong antigen-dependent binding to cancer cells, showcasing high cell avidity. The G9 CAR design effectively also prevented adherence to healthy cells reducing the risk of toxicity in a clinical setting. This novel low-affinity/high-avidity strategy led to superior antitumor efficacy in vitro and in vivo. In contrast, the first-generation G250 clinical CAR, known for its high affinity, was unexpectedly demonstrated to bind with high avidity to healthy cells, explaining the serious toxicities reported during initial clinical trials.
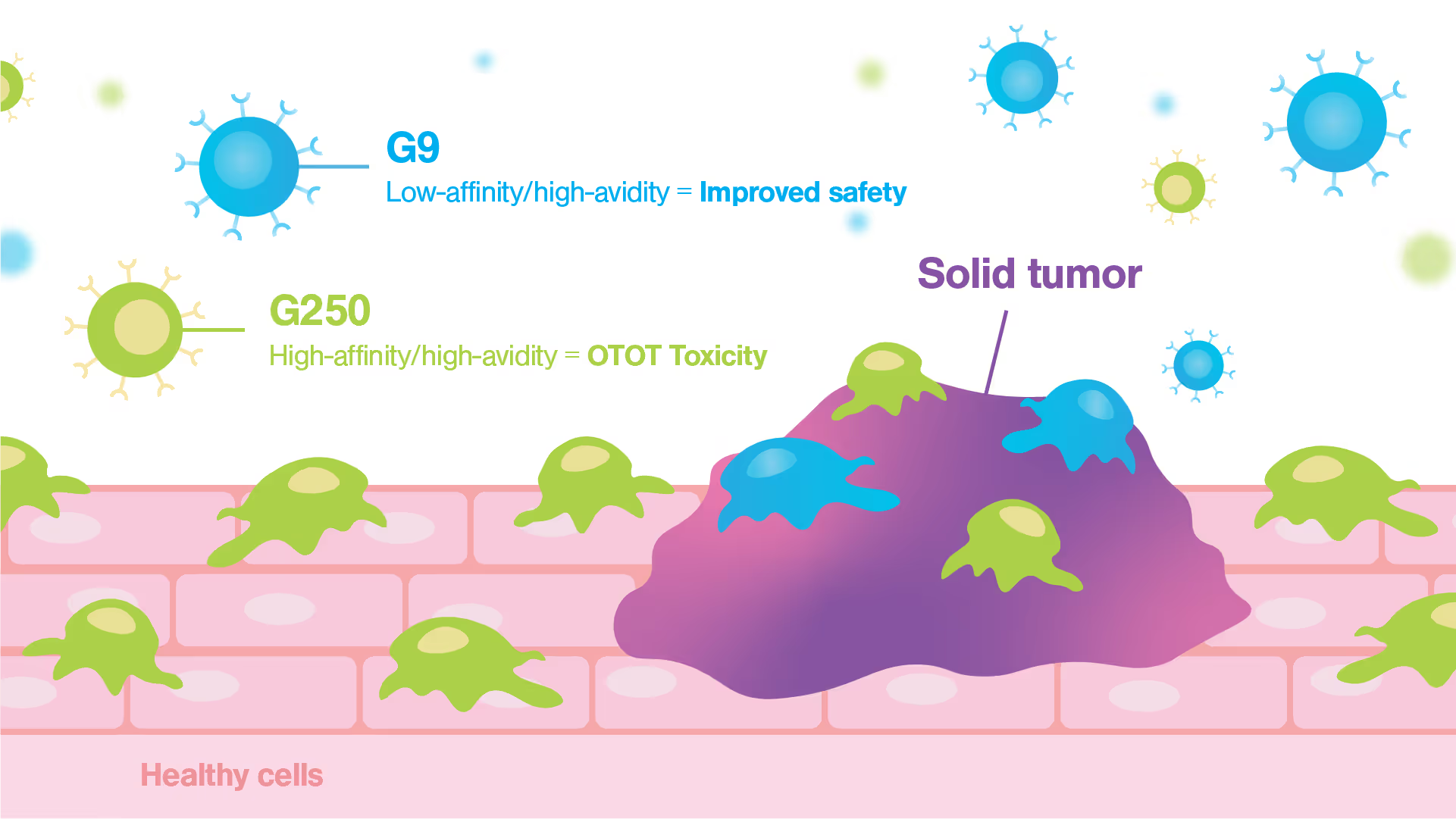
Quantifying the cellular interactions between different CAR designs and tumor and healthy cells was crucial to identify the mode of action and explaining why the avidity/affinity tuned G9 anti-CAIX CAR did not kill low antigen expressing healthy cells while retaining potent antitumor function. This discovery not only highlights the efficacy of the G9 CAR but also emphasizes the importance of assessing cell-cell interactions based on cell avidity metrics as a critical step for streamlining the selection process for safer and more effective CAR-T therapies.
“Carbonic anhydrase IX (CAIX) was considered undruggable as it is expressed in both tumor and healthy tissues. Fine-tuning the cell avidity of the anti-CAIX chimeric antigen receptor (CAR) T cells ensures CAR-T only kills CAIX high tumor cells but not CAIX low cholangiocytes, thus mitigating the on-target off-tumor side effects.”
Dr. Yufei Wang, Group Leader at Dana-Farber Cancer Institute
How does cell avidity play a critical role in improving CAR T selection in both liquid and solid tumors?
The field of cell therapy has heavily relied on traditional in vitro endpoint assays, such as cytokine secretion and cell killing, during preclinical development. These conventional assays yield valuable data; however, a staggering 83% of CAR-T candidates still fail post-preclinical development which not only prolongs the iterative development process, but also significantly inflates costs with a high failure rate in clinical trials. Cell avidity is a new parameter that has only recently been available to researchers to improve decision-making in selecting the most efficacious candidates to treat both liquid and solid tumors. Recent studies have used cell avidity in their preclinical workflow to advance therapies targeting multiple myeloma, non-Hodgkin lymphoma, and lung adenocarcinomas. Integrating traditional in vitro assays with cell avidity analysis has been instrumental in bolstering confidence, shortening R&D iteration cycles, and selecting candidates with improved potency and safety in preclinical models.










