Keeping up with the complexity of binding mechanisms
- Generate direct, physiologically relevant measurements of binding in its full, dynamic complexity
- Understand the mechanism of action of your therapeutic products by revealing its complex binding dynamics
- Balance potency and safety by optimizing binding
Cell Avidity enables potent and safe CAR-T therapies for solid tumors
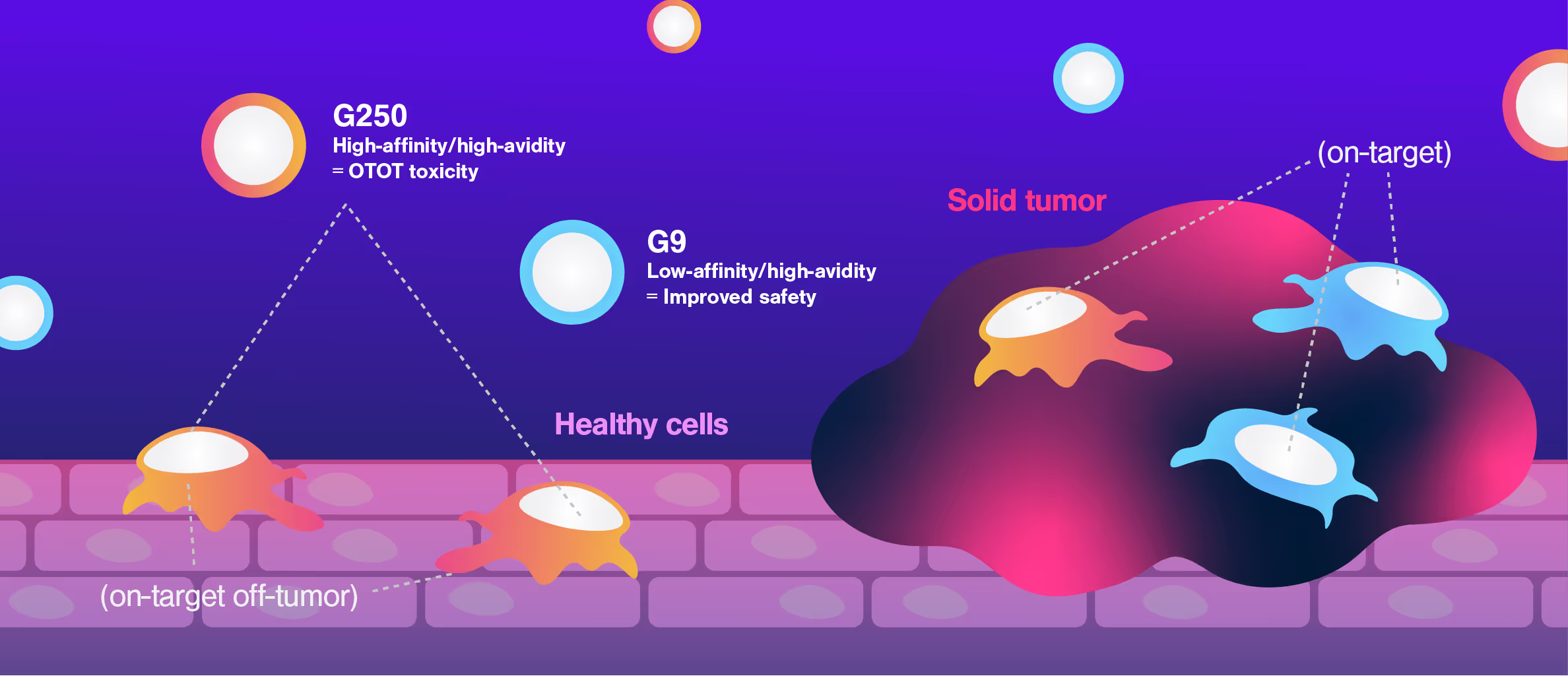
CAR-T therapies face a major challenge when targeting solid tumors: killing tumor cells effectively while sparing healthy cells that express the same target antigens, avoiding On-Target Off-Tumor toxicity.
Researchers at Dana-Farber Cancer Institute used Cell Avidity assays to evaluate CARs targeting CAIX, a protein found in both kidney tumors and healthy bile duct tissue.
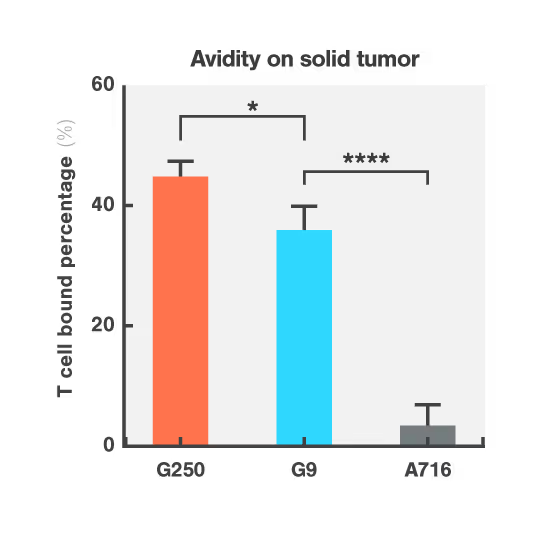
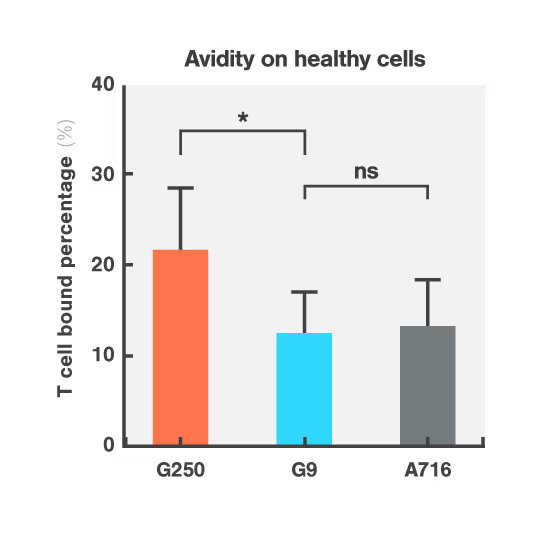
The G250 CAR showed high avidity on both cancerous and healthy cells, mirroring the in vivo results that led to G250 being withdrawn from the clinic. On the contrary, the G9 CAR showed high avidity for the cancerous cells, but low avidity for healthy bile duct cells, consistent with a higher safety profile.
Figures: Cell Avidity of CAR-T cells on tumor skrc-59 cells (Figure 1) and normal MMNK-1 cholangiocytes (Figure 2) at 1000 pN endpoint. The normalized percentage of binding is defined by minus the binding of UNT. Data are mean ± SD (n = 4 per group). P values are defined by unpaired two-tailed t-tests (*p < 0.05; ****p < 0.0001).
Dive into the publication
Affinity fine-tuning anti-CAIX CAR-T cells mitigate on-target off-tumor side effects
How Cell Avidity could have predicted a clinical trial failure
Despite promising preclinical data, the APRIL CAR failed in a Phase I clinical trial. This is the case for more than 80% of CAR-T candidates, highlighting that current in vitro and in vivo preclinical assays have insufficient power to select potent drugs.
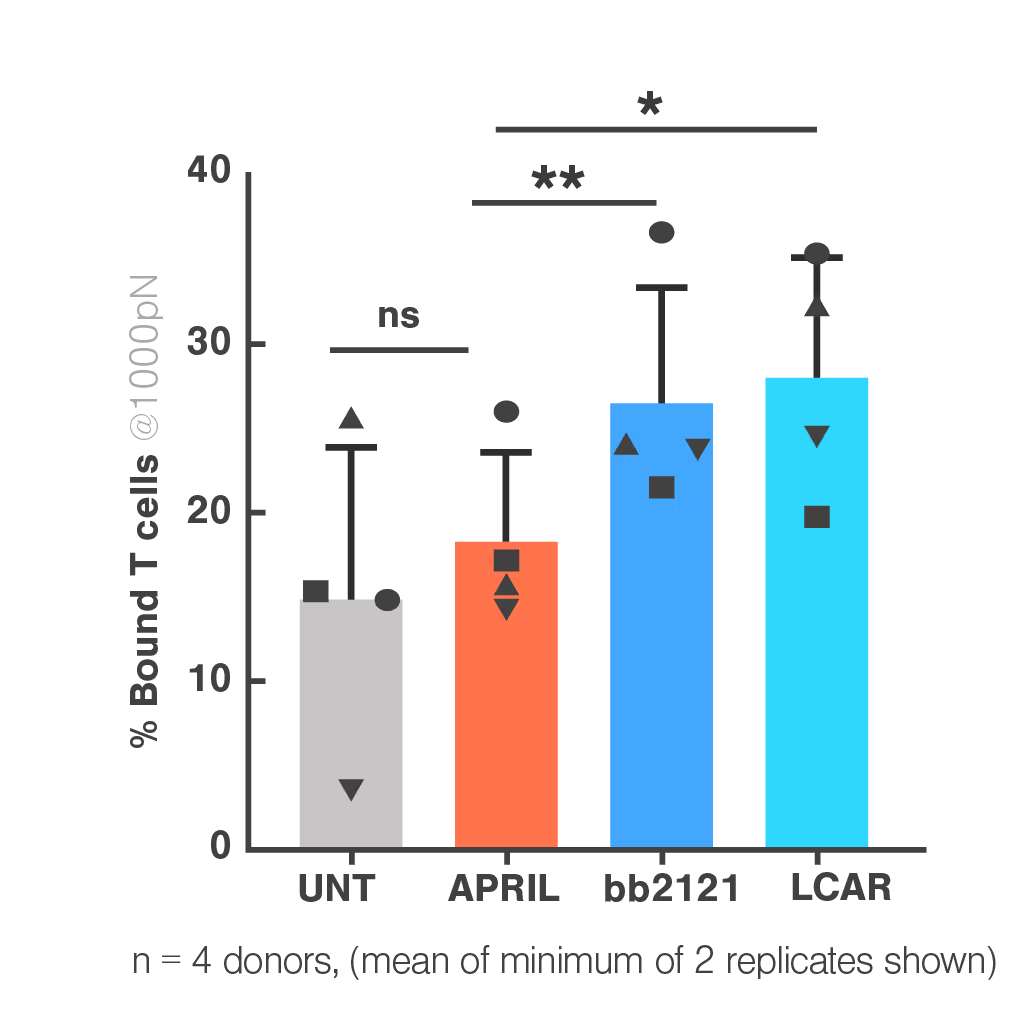
Researchers at the University College London performed a retrospective study of APRIL-CAR including Cell Avidity analyses, which provide a comprehensive measurement of all interactions at the interface of an immune cells and a cancer cell. APRIL CAR showed no significant binding to tumor cells compared to the negative controls. This poor performance was related to inadequate CAR expression, which conventional methods failed to detect.
Cell Avidity analyses could have revealed this early in the development process, improving candidate selection and potentially avoiding the APRIL CAR trial failure.
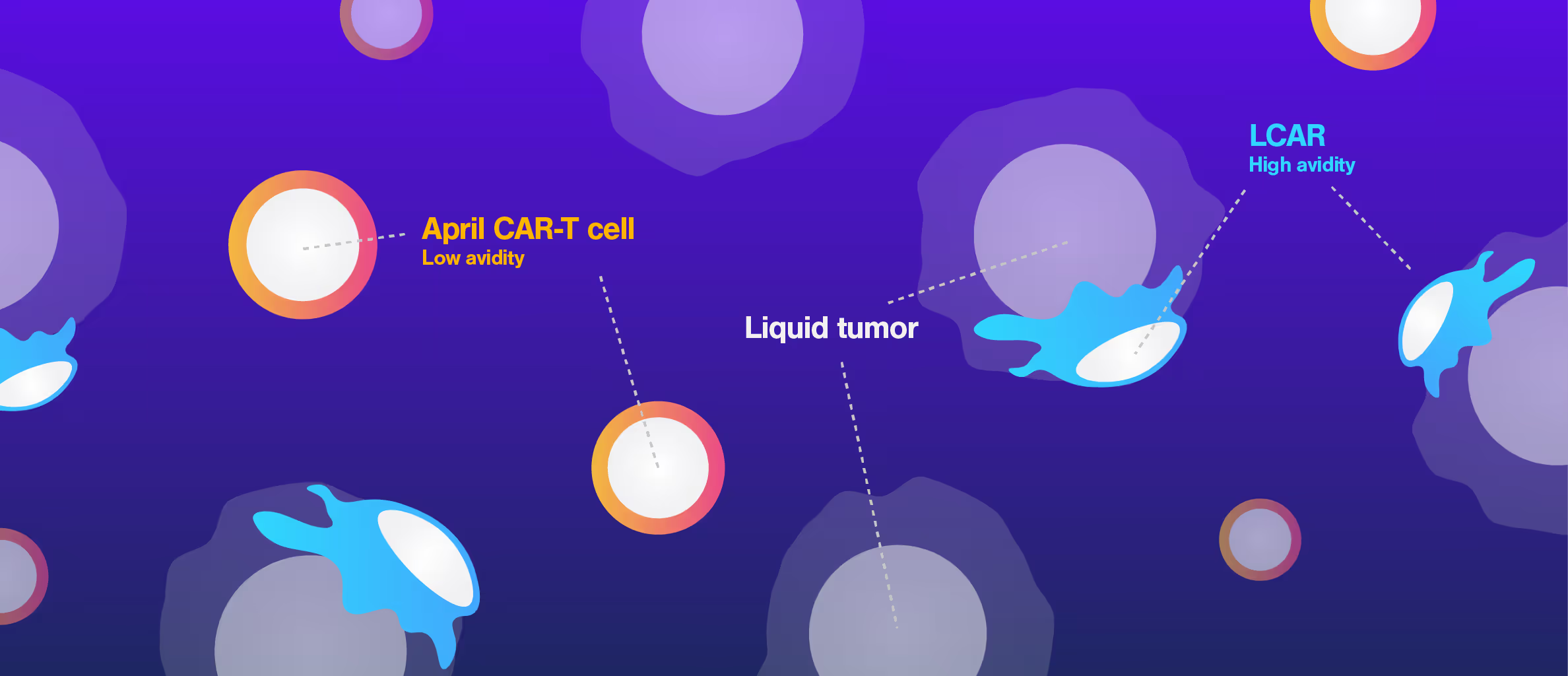
Figure 1: In the cell-cell context, APRIL CAR binding to BCMA-expressing target cells is indistinguishable from the untransduced control and lower than both FDA-approved clinical CARs (bb2121 and LCAR). Bar plot shows percentage bound CAR T cells from 4 healthy donors against myeloma cell line H929, after 1000 pN force application. Multiple paired T tests and Holm Sidak correction *p<0.05, **p<0.01, ***p<0.001.
Dive into the publication
Limited efficacy of APRIL CAR in patients with multiple myeloma indicate challenges in the use of natural ligands for CAR T-cell therapy
Harvard Medical School uses Cell Avidity to overcome CAR resistance mechanism
A common challenge in CAR T development is that standard in vitro assays often fail to predict in vivo performance. CARs can show strong killing in cell-based tests but still underperform in animal models or clinical settings, leaving researchers with limited insight into the underlying mechanism.
At Harvard Medical School, researchers encountered this exact issue while developing a CD27-based CAR T therapy. Although in vitro data looked promising, in vivo efficacy was poor. Using Cell Avidity analysis, they discovered that a cleavage site in the CAR reduced surface expression, weakening binding to tumor cells and explaining the drop in performance.
This insight clarified the disconnect between in vitro and in vivo data. Cell Avidity pinpointed the mechanism of failure, helping the team select the optimal CAR for further development.
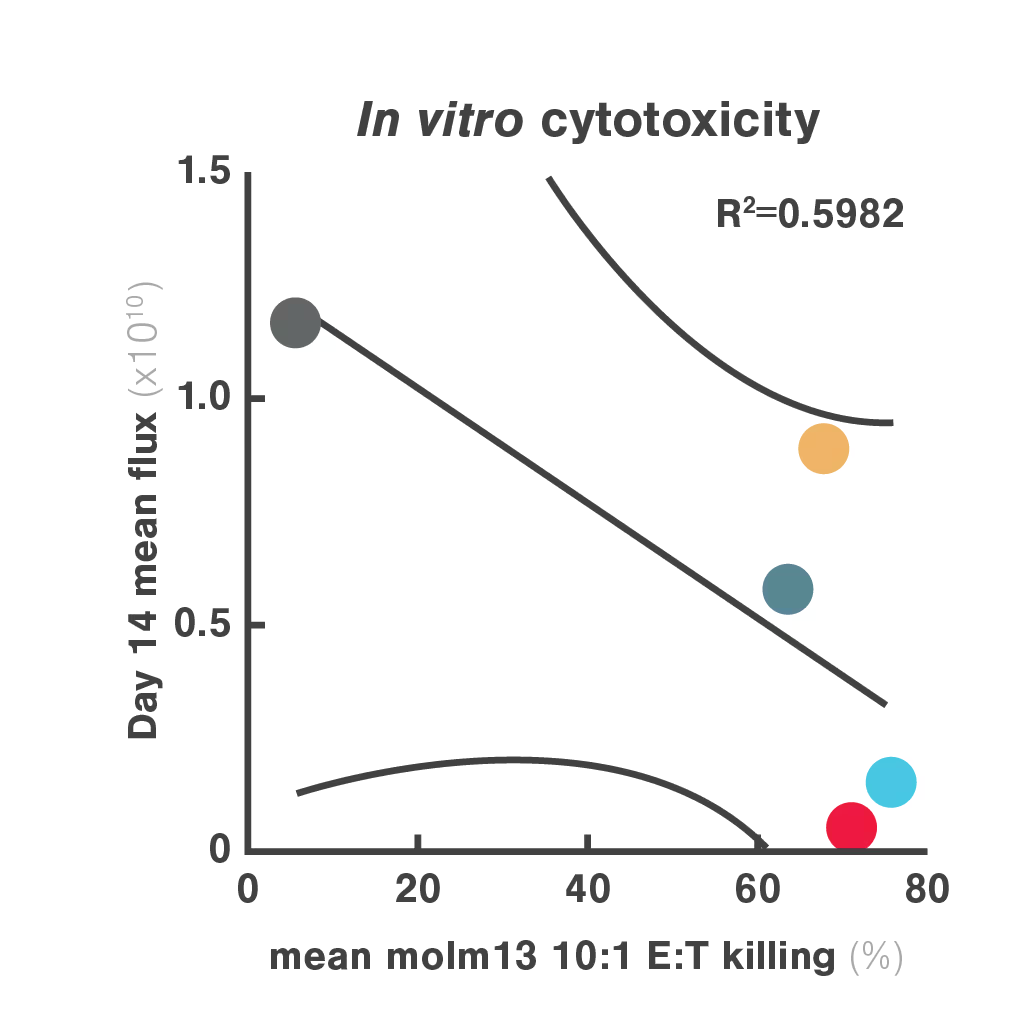
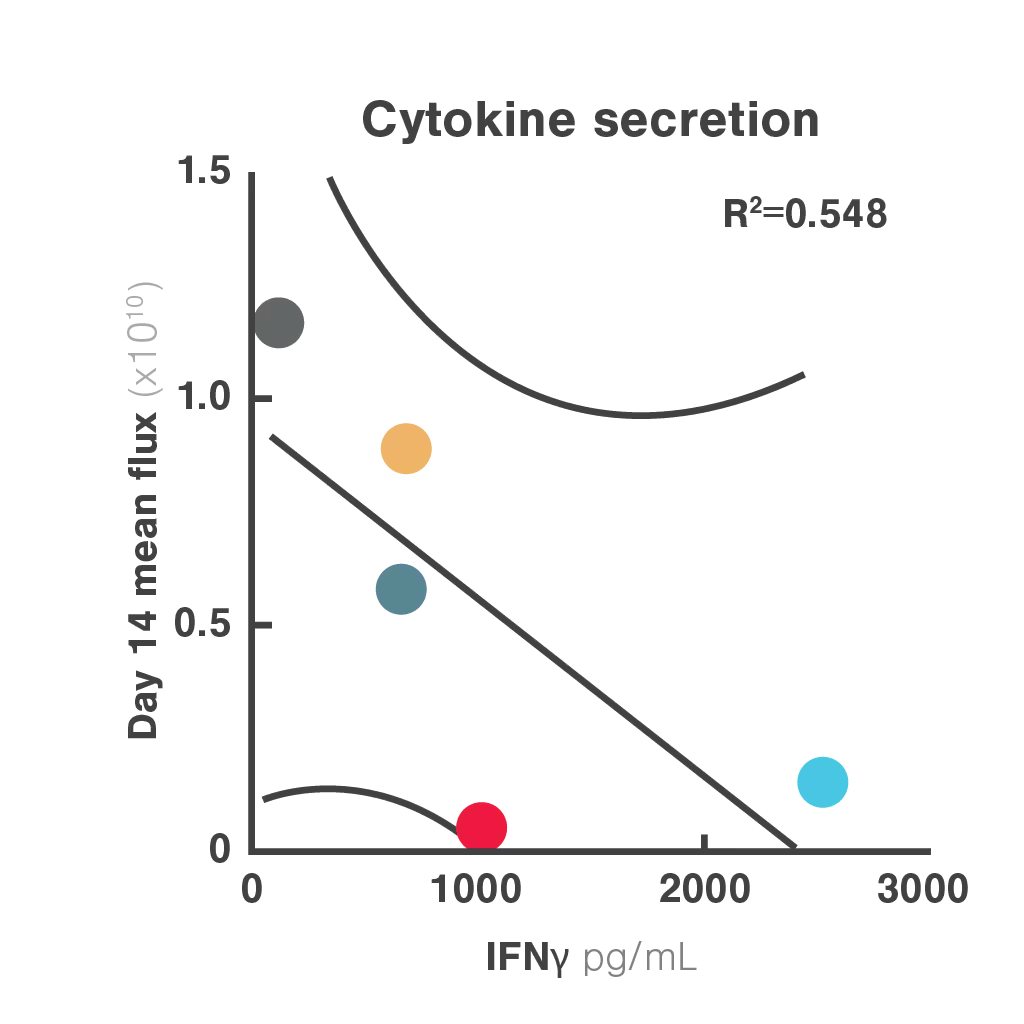
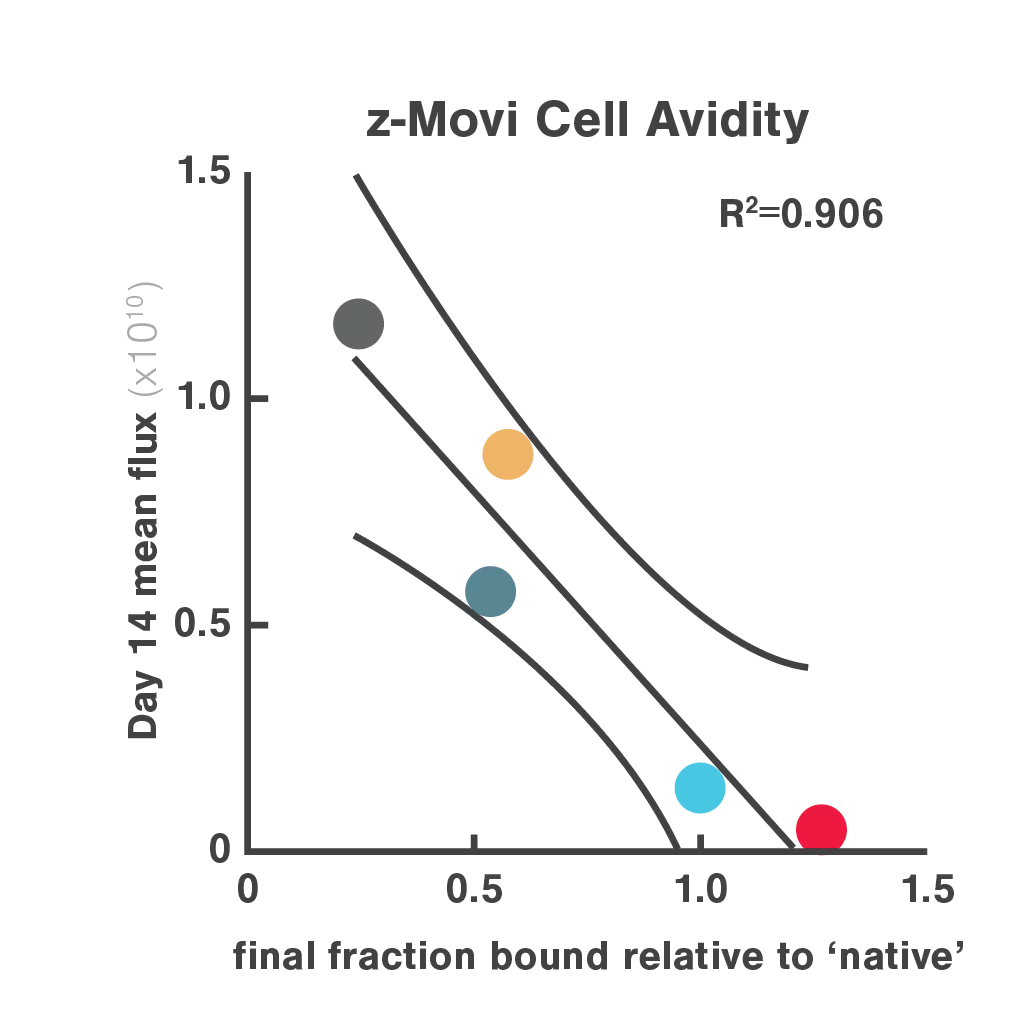
Figures: Comparison of three in vitro CAR-T potency metrics showed that cell avidity best predicted in vivo anti-tumor effect (R² = 0.906), outperforming cytotoxicity (R² = 0.5982) and cytokine secretion (R² = 0.548).
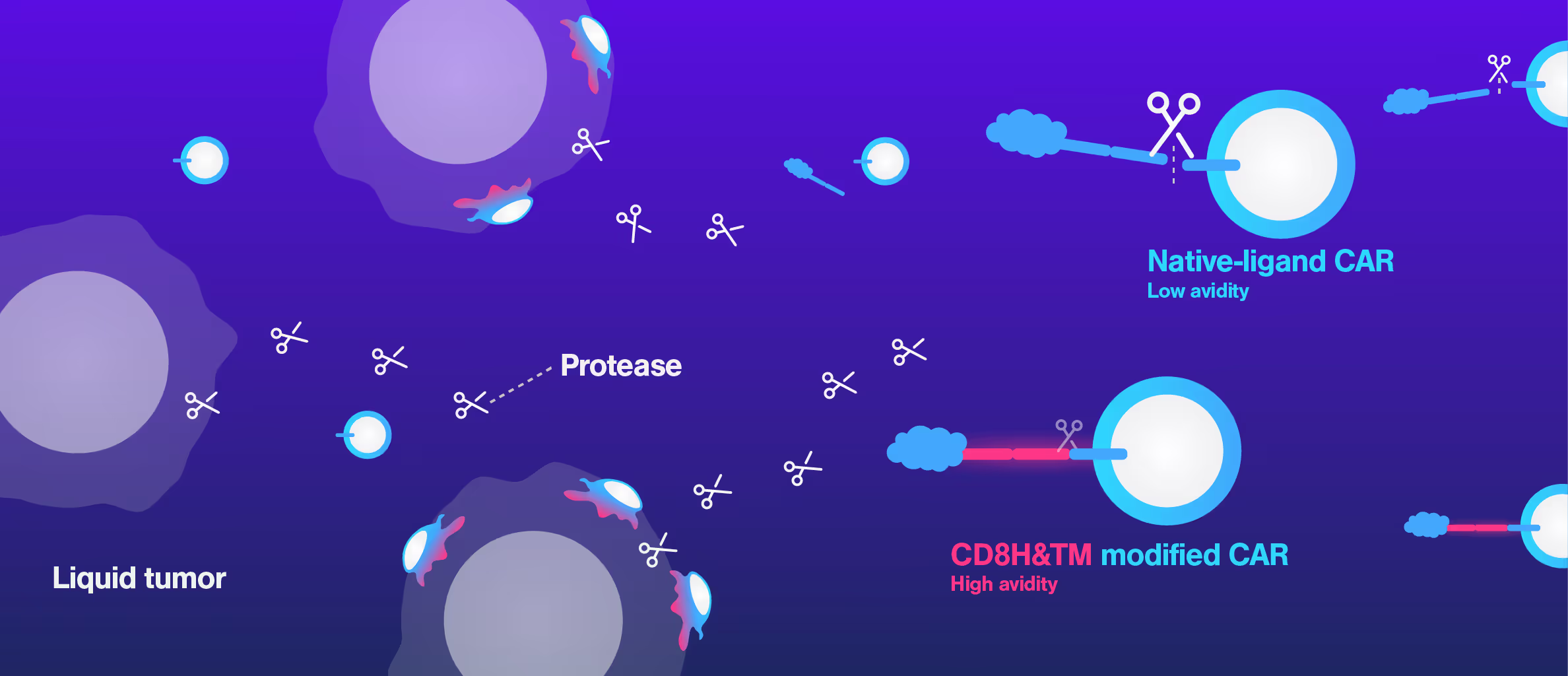
Dive into the publication
Non-cleavable hinge enhances avidity and expansion of CAR-T cells for acute myeloid leukemia
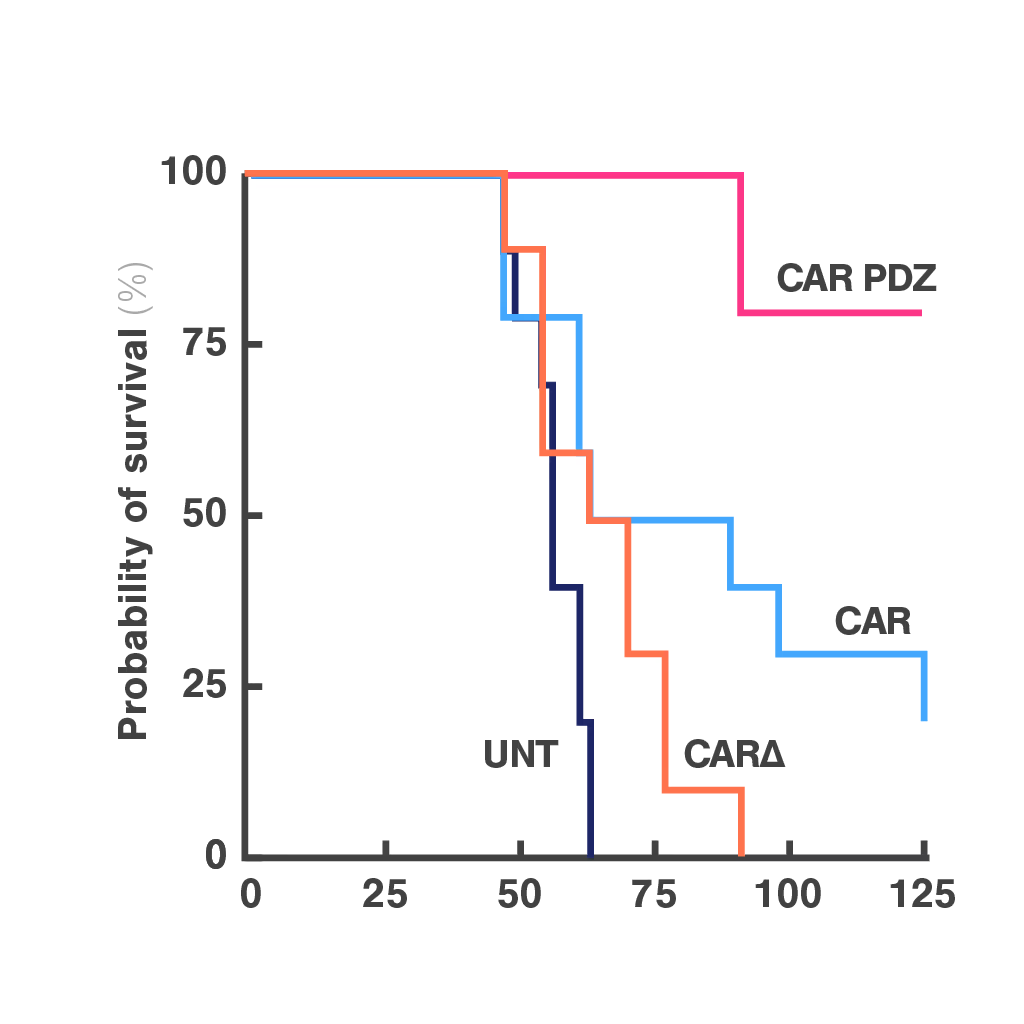
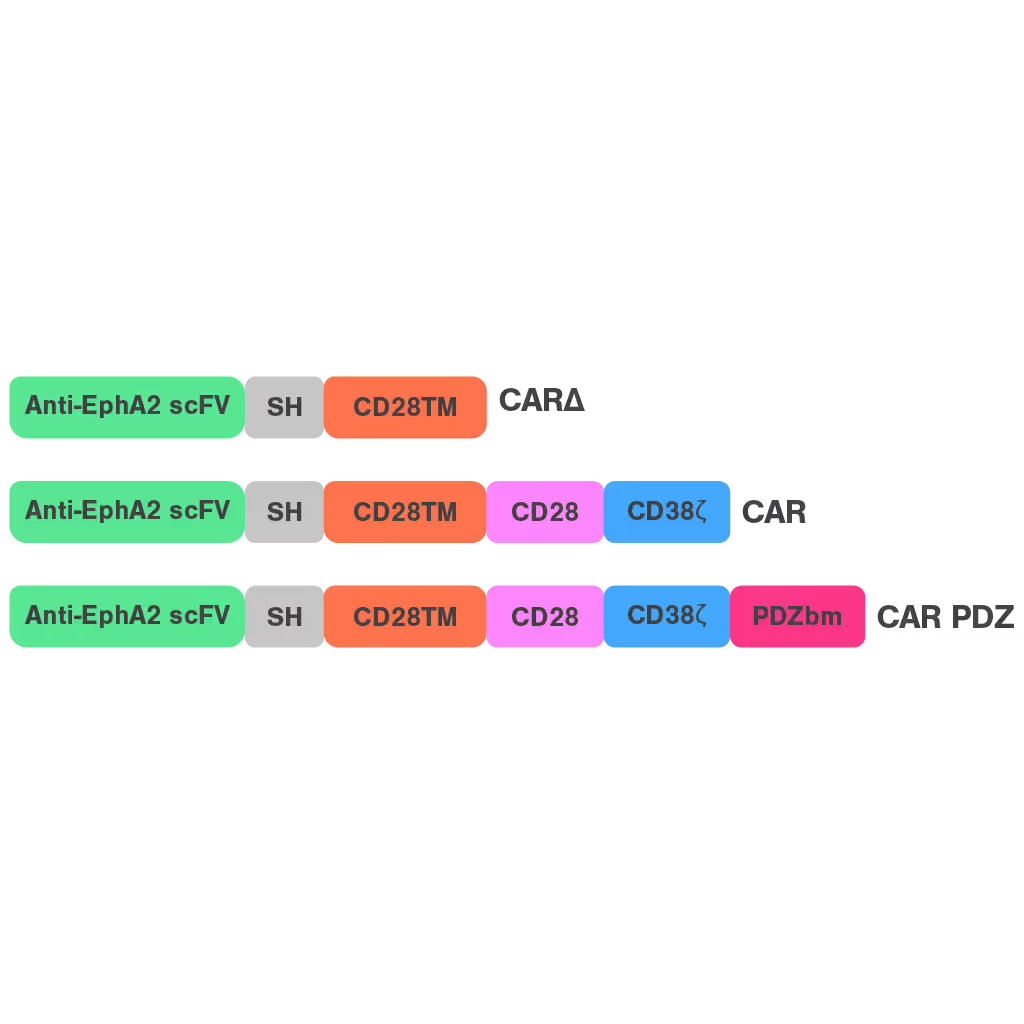
Cell Avidity quantifies the impact of immune synapse engineering
CAR-NK therapies often face limited efficacy due to weak and disorganized immune synapses. Traditional assays cannot capture the quality of these cell interactions, making it hard to evaluate design changes or predict performance.
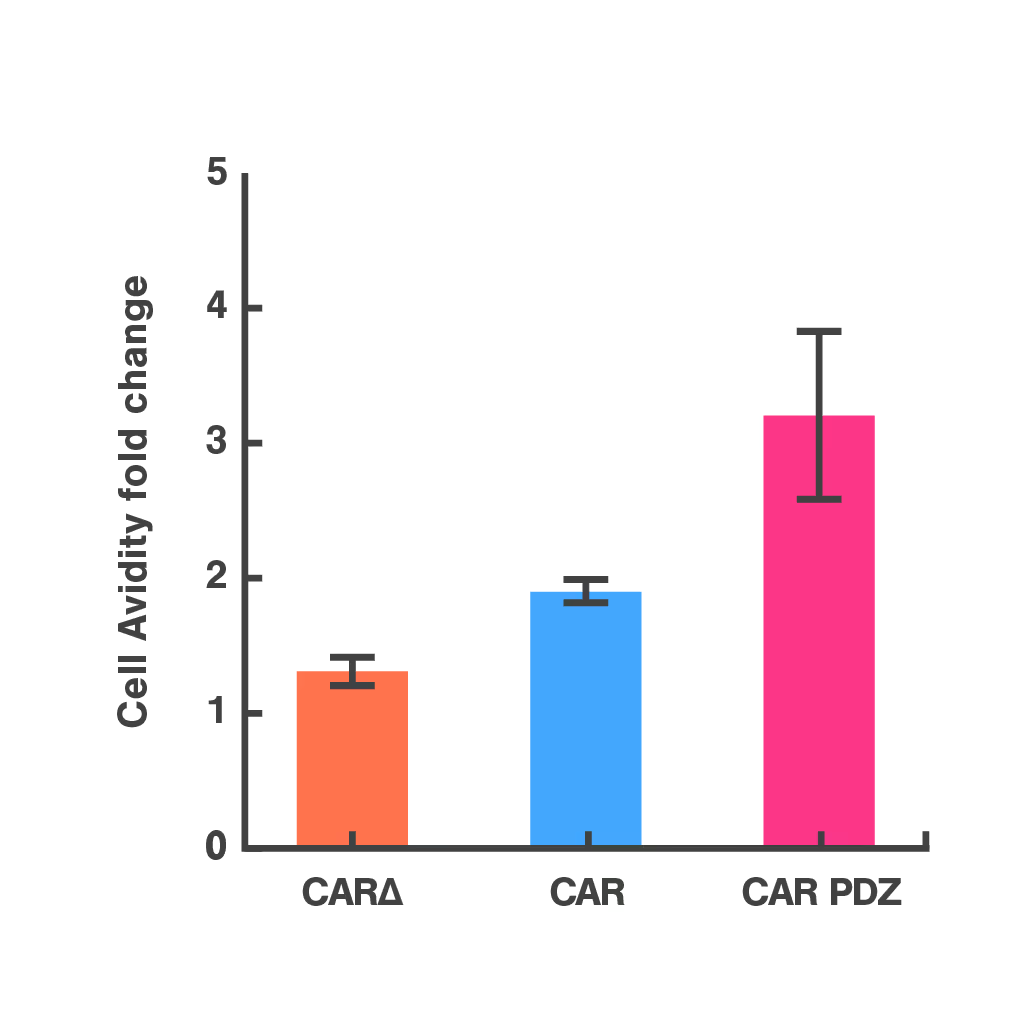
To address this, researchers at St. Jude Children's Research Hospital modified the CAR structure by adding a protein interaction domain (PDZ) to enhance synapse formation. Using cell avidity analysis, they observed stronger binding to tumor targets and improved tumor control in mouse models.
These findings were supported by immune synapse imaging, with cell avidity offering a faster and more reproducible measure of functional improvement.
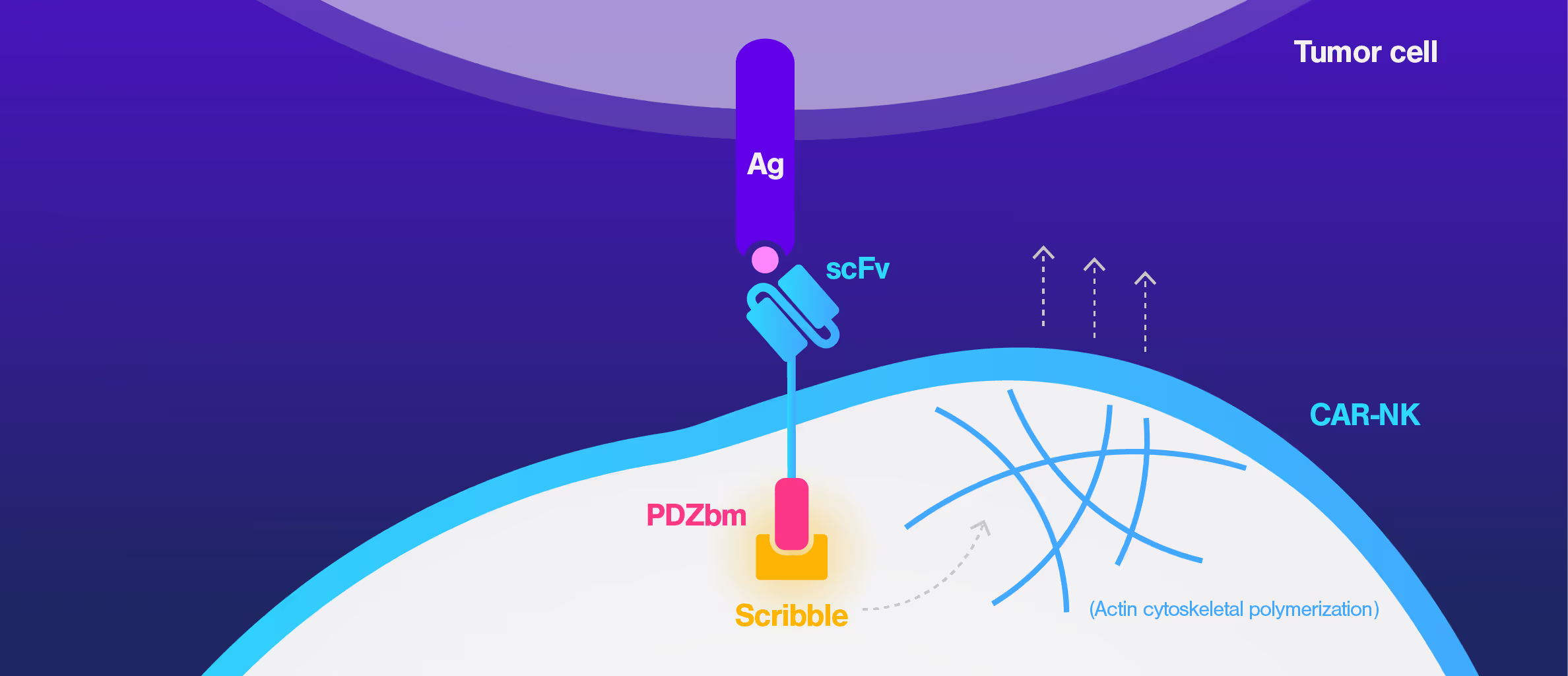
Dive into the publication
Synapse-tuned CARs enhance immune cell anti-tumor activity
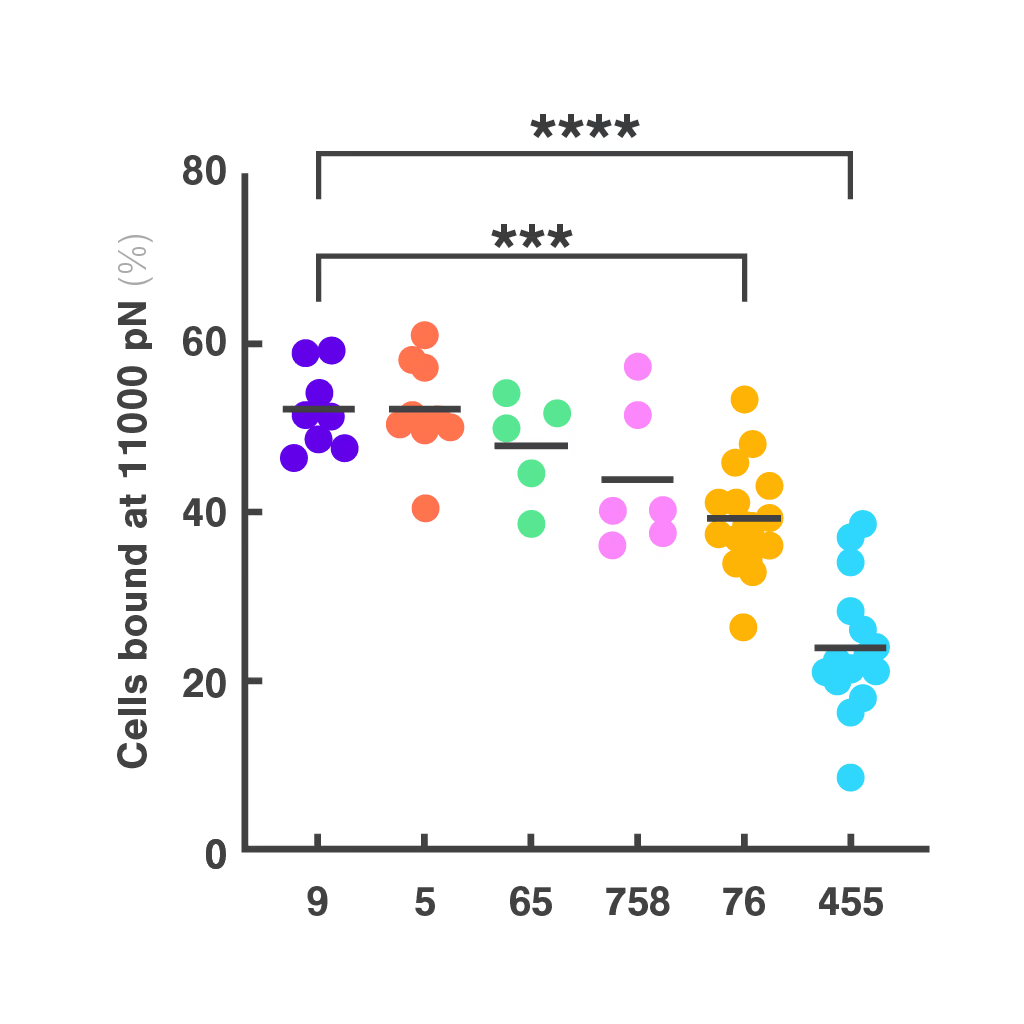
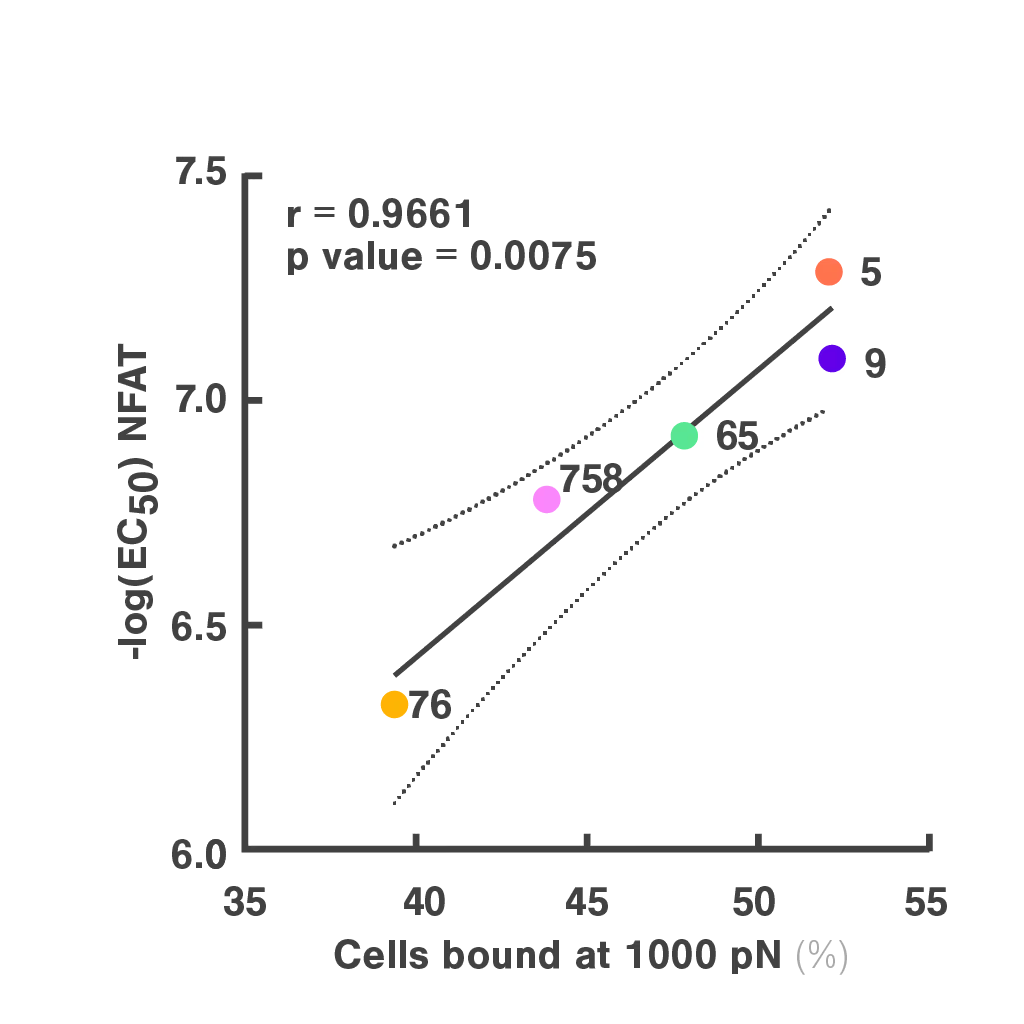
Cell Avidity improves TCR selection
Choosing the right TCRs for therapy is challenging. Standard assays like peptide sensitivity and killing tests are time-consuming and often fail to predict clinical performance, especially in personalized treatments targeting unique tumor antigens.
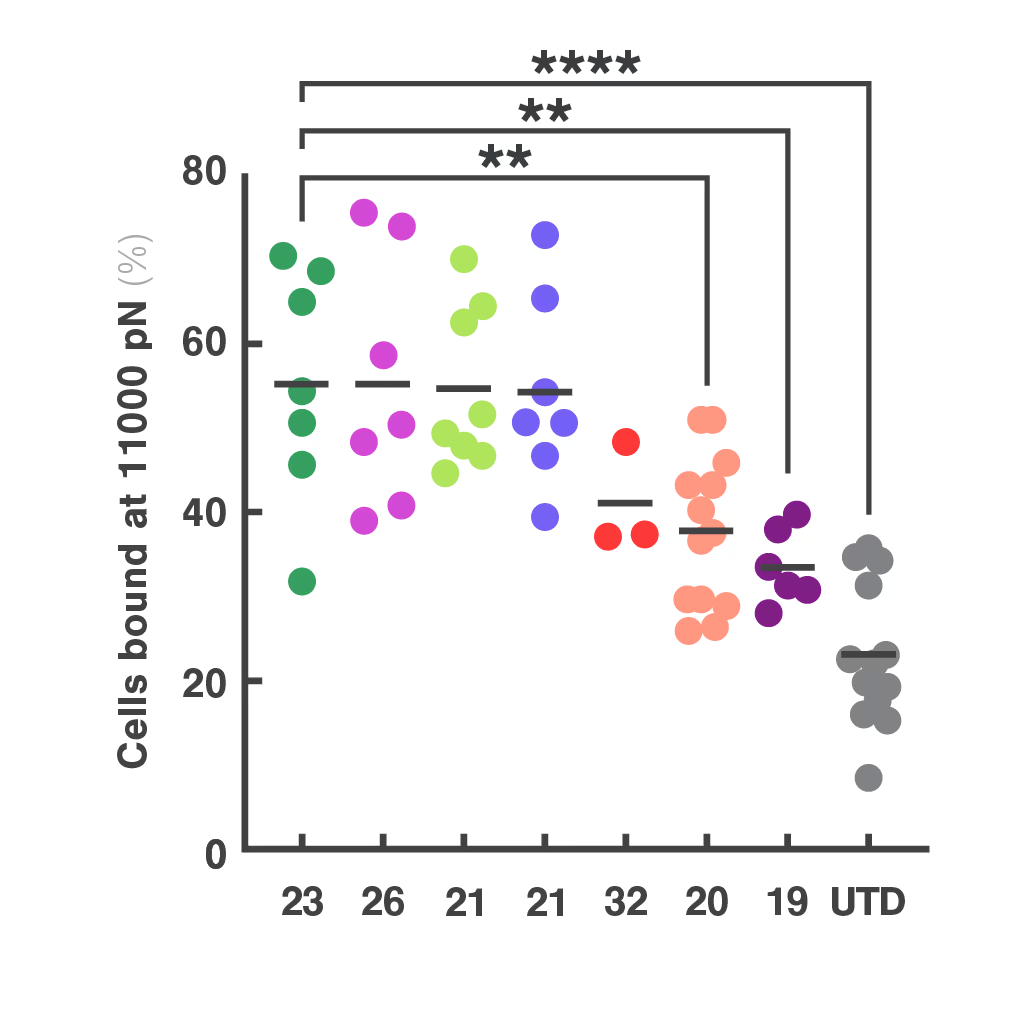
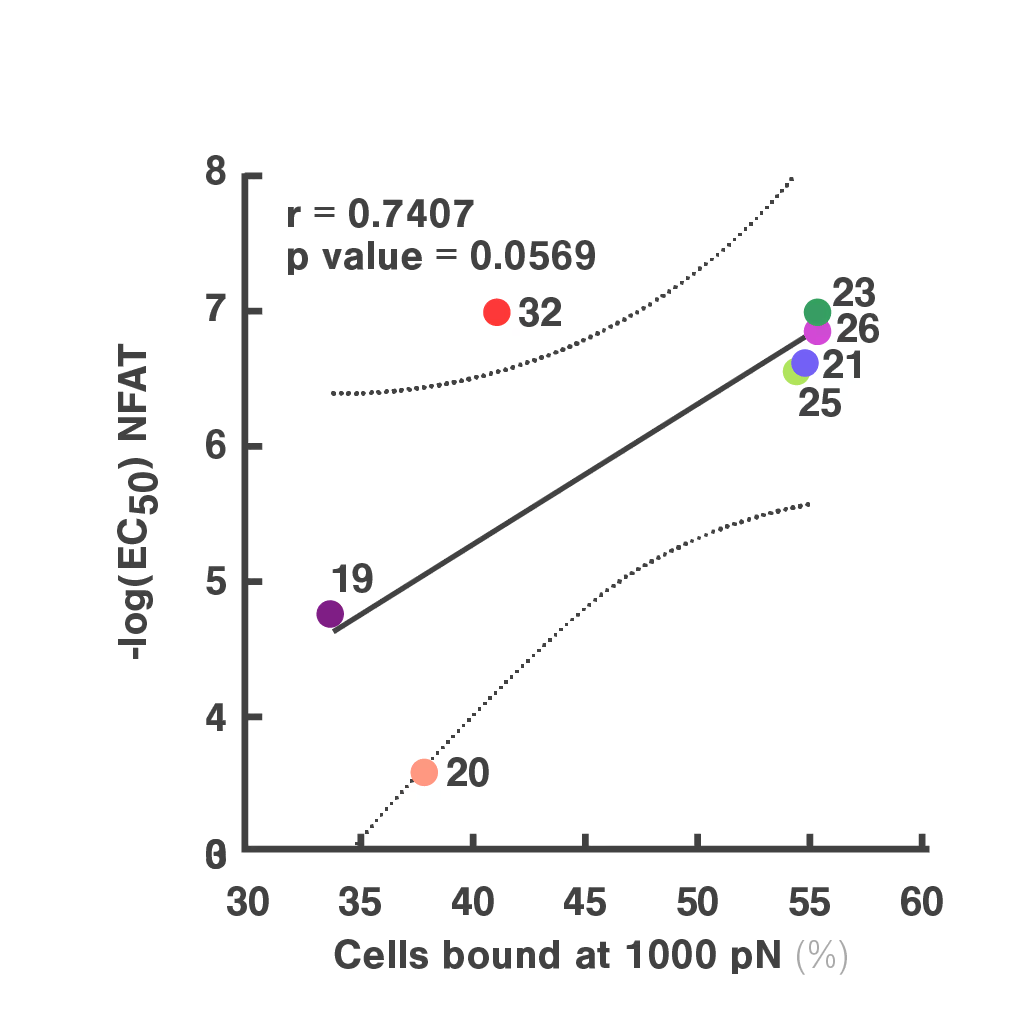
Researchers at the Technical University of Munich explored cell avidity as a better predictor of TCR function. Using a Jurkat-based TCR system, they found that cell avidity closely correlated with functional performance, clearly distinguishing strong TCRs from weak ones.
This approach offered faster, more accurate screening and helped reduce false positives and negatives that commonly delay development.
Figures: Cell avidity predicted in vitro functionality of SARS-CoV-2 TCRs (1, 2) and tumor neoantigen-specific TCRs (2, 3). Bar plots show cell avidity at 1000 pN endpoint (mean shown as line; **p < 0.01, ***p < 0.001,****p < 0.0001). Left, Pearson correlation between cell avidity and NFAT EC50.
Dive into the publication
Advances in preclinical TCR characterization: leveraging cell avidity to identify functional TCRs
Avidion
The next generation Cell Avidity platform


Avidigo
White glove Cell Avidity services

z-Movi
For small sized Cell Avidity studies
These cards are NOT components because they use the finsweet nested collection logic. To pull in the posible multiple people wo worked on it.
This type of 1-many relation is not supported native in Webflow.
Also this section is hidden when emtpy. To keep everything visible here, that is being done outside the webflow designer from within Slater.
Harnessing the CD2 axis to broaden and enhance the efficacy of CAR T-cell therapies
CD4+ T cells mediate CAR-T cell-associated immune-related adverse events after BCMA CAR-T cell therapy
Chimeric antigen receptor T cells against the IGHV4-34B cell receptor specifically eliminate neoplastic andautoimmune B cells
SITC 2025
CAR-TCR Summit 2025
CICON 2025