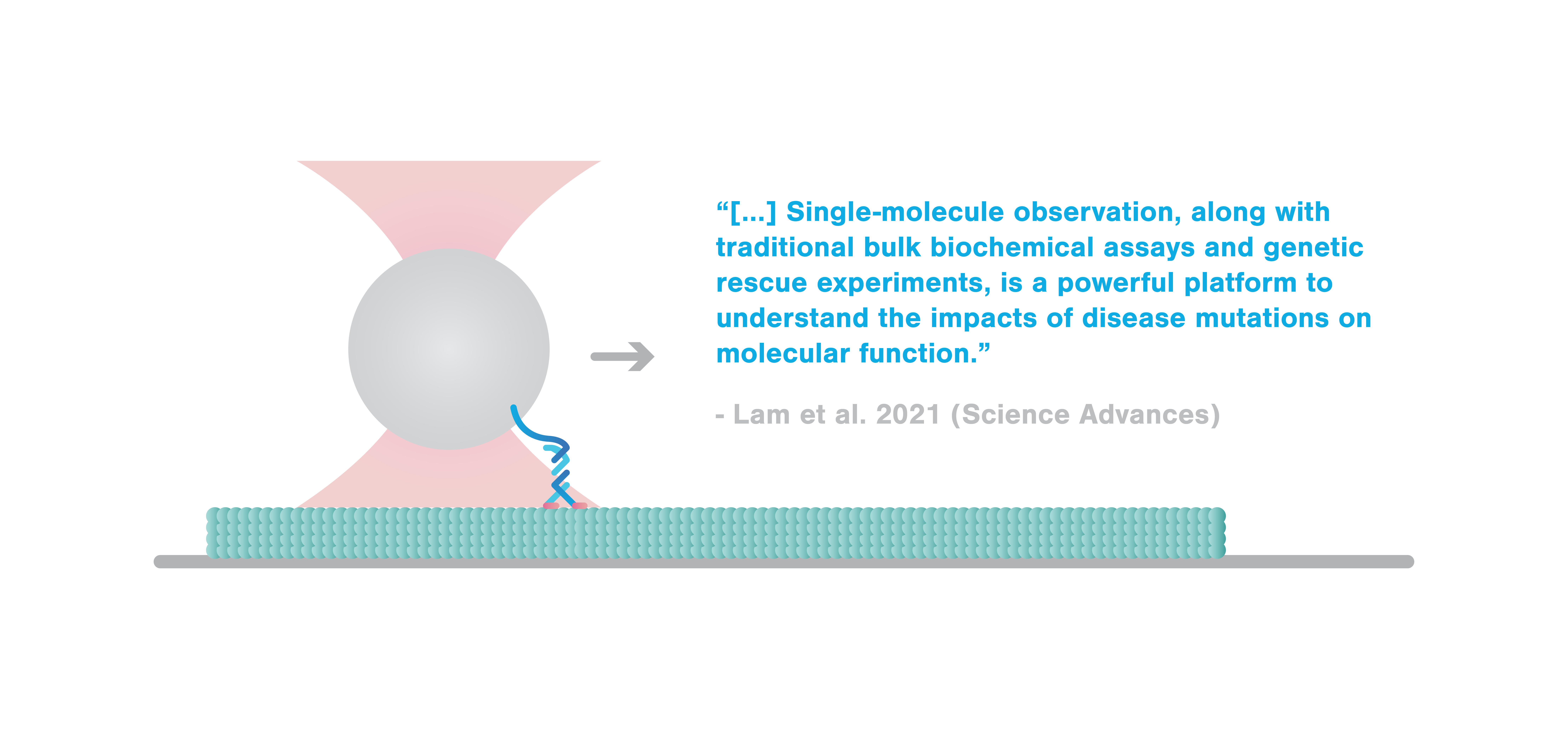
In a recent article published in Science Advances, researchers investigated the mechanical consequences of a missense mutation within a highly conserved region, P305L, in the kinesin motor family member A1 (KIF1A). By employing a single-molecule approach using the C-Trap® as well as biochemical and genetic assays, Lam et al. found that the mutation highly affects the microtubule-associated rate of the motor, impacting force generation and movement under resistive loads.
Kinesins are molecular motor proteins that carry cargo along the microtubules within cells. KIF1A belongs to the large kinesin-3 (KIF1) family that executes long-distance transports along the axons of neurons. Within KIF1A, over 100 mutations are known to cause KIF1A-associated neurological disorder (KAND), making it crucial to understand the consequences of these mutations on motor function and help develop efficient therapeutic treatments.
Lam et al. used C-Trap optical tweezers to determine whether the mutation affects the force generation ability of the KIF1A motor that is important for transporting cargo along microtubules. Due to its nanometer precision, the C-Trap enables measurements of the individual steps of the motor and allows the application of pico-Newton (pN) stall forces imitating cargo load.
The performed experiments revealed that the mutated KIF1AP305L detaches from the microtubule at an about 4-fold reduced force in comparison to the wild-type KIF1A. Similarly, another mutated motor was tested, KIF1BP276L, and showed a faster detachment by about 50% in comparison to its wild-type variant. These findings support the results from TIRF microscopy analysis, whereby the researchers observed that the mutation affects the ability of the kinesin motor to bind to the microtubules.
“Here, we use single-molecule methods to dissect the specific defects in an understudied pathological variant of KIF1A, P305L, demonstrating that single-molecule observation, along with traditional bulk biochemical assays and genetic rescue experiments, is a powerful platform to understand the impacts of disease mutations on molecular function.“ – the authors conclude.
Read the full article to discover all results presented by Lam et al., published in the journal Science Advances under the title “A highly conserved 310 helix within the kinesin motor domain is critical for kinesin function and human health”.